Andhra Pradesh BIEAP AP Inter 2nd Year Zoology Study Material Lesson 4(b) Immune System Textbook Questions and Answers.
AP Inter 2nd Year Zoology Study Material Lesson 4(b) Immune System
Very Short Answer Questions
Question 1.
Define the terms immunity and immune system.
Answer:
Immunity :
It is the ability of the host or individual to fight against the disease-causing organisms that is called immunity.
Immune System :
The network of organs, cells, and proteins that protect the body from harmful, infectious agents such as bacteria, viruses, animal parasites, fungi, etc., is called the immune system.
Question 2.
Define the non-specific lines of defence in the body.
Answer:
Non-specific lines of defence is the first line of defence mechanism and are also called as innate immunity, which is inherited by birth. It does not depend on prior contact with the microorganism. Non-specific lines of defence mechanism executed by four barriers namely;
- Physical barriers
- Physiological barriers
- Cellular barriers
- Cytokine barriers.
Question 3.
Differentiate between mature B-cells and functional B-cells.
Answer:
| Mature B-cells | Functional B-cells |
| 1. B-cells arise from stem cells and develop into mature B-cells. | 1. Functional B-cells develop from mature B-cells. |
| 2. The mature B -cells express antibodies on their surface to bind and engulf antigen for processing and presenting. | 2. Functional B-cells differentiate into memory and plasma cells. Plasma cells produce antibodies, to eliminate antigen. |
Question 4.
Write the names of any four mononuclear phagocytes.
Answer:
- Histocytes – present in the connective tissue
- Kupffer cells – in the liver
- Microglia – in the brain
- Osteoclasts – in the bone.
![]()
Question 5.
What are complement proteins?
Answer:
Complement proteins are a group of inactive plasma proteins and cell surface proteins. They are activated in cascade fashion. When activated, they form a membrane attack complex (MAC) that forms a pore in the plasma membrane, allowing ECF to enter the cell and make it swell and burst.
Question 6.
Colostrum is very much essential for the newborn infants.
Answer:
The colostrum secreted by the mother during the initial days of lactation has abdundant IgA antibodies to protect infant from initial sources of infection. .
Question 7.
Differentiate between perforins and granzymes.
Answer:
Perforins :
Perforins are the enzymes produced during the process of cell mediated immunity from cytotoxic T-lymphocytes. Perforins form pores in the cell membrane of the infected cells.
Granzymes:
Granzymes are the enzymes produced during the process of cell mediated immunity from cytotoxic T-lymphocytes. Granzymes enter th6 infected cells through the perfororations and activate certain proteins which help in distinction of the infected cell i.e., called apoptosis.
Question 8.
Explain the mechanism of Vaccinization (or) Immunization.
Answer:
Vaccinization is based on property of the mempry of the immune system. During the process of vaccinization, inactivated or weakend pathogens or antigenic proteins of pathogen * are introduced into the body of the host and they initiate the production of antibodies and also generate memory B-cells and memory T-cells. On subsequent exposures, the memory cell recognizes that pathogen quickly and overcomes the invader with a rapid and massive production of antibodies.
Question 9.
Mention the various types of immunological disorder.
Answer:
There are various types of immunological disorders.
- Immuno deficiency disorders
- Hypersensitivity disorders
- Antoimmune disorders
- Graft rejection.
Question 10.
More and more people in metro cities of India are prone to allergies. Justify.
Answer:
The people in metro cities of India suffer from allergies leading to asthmatic attacks due to environmental pollutants.
Question 11.
What are auto-immune disorders? Give Any two examples.
Answer:
Generally our immune system can recognize our own proteins (self antigens) and does not attack our own tissues. Unfortunately, in some cases our immune system fails to recognise some of our own body proteins and treats them as foreign antigens, that results in attacks on our own tissues. This leads to some very serious diseases collectively known a autoimmune disease.
Eg: 1. Graves’ disease 2. Rheumatoid arthritis.
![]()
Question 12.
How can the graft rejections be avoided in patients?
Answer:
After organ transplantation our body recognises them as foreign and initiate the graft rejection To avoid this tissue and maching and blood group matching are essential before undertaking graft. Even after this the patient has to take immuno-suppressant ‘drugs throughout the life.
Short Answer Questions
Question 1.
Write short notes on B-cells.
Answer:
The lymphocytes capable of producing antibodies and can capture circulating antigens are called B-cells. They are produced from the stem cells in the bone marrow, liver of foetus and bursa of fabricius in birds. Mature B-cells express or display Ig M and Ig D antibodies on their membrane surfaces. As these antibodies can take antigens, the mature B-cells are also called immuno-competent B-cells.
In secondary lymphoid organs these immune-competent B-cells develop into functional immune cells which later differentiate into long lived memory cells and effector plasma cells. The plasma cells produce antibodies specific to the antigen to which they are exposed. Memory cells store information about the specific antigens and show quick response, when the same type of antigen invades the body later.
Question 2.
Write short notes on Immunoglobulins.
Answer:
Whenever pathogen enters our body, the B-lymphocytes produce an army of proteins called antibodies to fight with them. They are highly specialised for binding with specific antigens. The part of an antibody that recognises an antigen is called the paratope antigen binding site.
Based on their mobility, antibodies are of two types.
1. Circulating or free antibodies :
These are present in the body fluids like serum, lymph etc.
2. Membrane bound antibodies :
These are present on the surface of the mature B-cells as well as the memory cells.
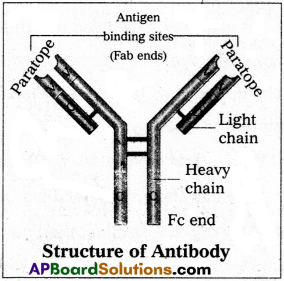
Structure :
Immunoglobulin is a Y’ shaped molecule with four polypeptide chains of which two &ye long identical heavy chains (H) and two are small, identical light chains (L). These two chains are linked by disulfide bonds. One end of the antibody molecule is called Fab end (Fragment- antigen binding) and the other end is called Fc end (Fragment-Crystaline). Based on the structure, the antibodies are of five types namely Ig G, Ig A, Ig M, Ig D and Ig E.
Question 3.
Describe various types of barriers of innate immunity.
Answer:
Innate immunity is a non-specific type of defence mechanism which provides the first line of defence mechanism against infections. This is executed by providing different types of barriers like;
a) Physiological:
Skin and mucus membranes are the main physical barriers. Skin prevents the entry of micro-organism, whereas the mucus membranes help in trapping the microbes entering our body.
b) Phyloigical barriers :
Secretions of the body like HCl in the stomach, saliva in the mouth, tears from the eyes are the main physiological barriers against microbes.
c) Cellular barriers :
Certain types of cells like polymorphonuclear leucocytes, monocytes, and natural killer cells in the blood as well as macrophages in the tissues are the main cellular barriers. They phagocytose and destroy the microbes-.
d) Cytokine barriers :
The cytokines secreted by the immune cells like interleukins and interferons are involved in differentiation of cells of immune system and protect the non-infected cells from further infection.
![]()
Question 4.
Explain the mechanism of humoral immunity.
Answer:
The immunity mediated by the antibodies that released into the fluids of the body (humors) such as plasma, lymph etc., is called humoral immunity.
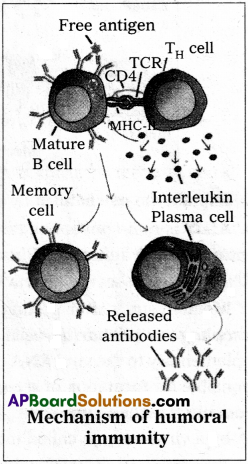
Mechanism of humoral immunity :
Whenever the antigen (exogenous) enters into our body, they reach secondary lymphoid organs, where the free antigens bind to Fab end of the membrane bound antibodies that are present on the surface of mature B-cells. They engulf and process antigen. Then they display the antigenic fragments on their membrane with the help of Class-II MHC molecule. Then appropriate T4 cells recognise them and interact with the antigen-MHC-II complex and release interleukins, which stimulates the B-cells to proliferate and differentiate into memory cells and plasma cells. The plasma cells release specific antibodies into plasma or extra cellular fluids.
These antibodies help in opsonising and immobi – lizing the bacteria, neutralizing and cross linking of antigens leading to agglutination of insoluble antigens and precipitation of soluble antigens. They also activate the phagocytes and complement system.
Question 5.
Explain the mechanism of cell mediated immunity.
Answer:
The immunity mediated by the activated T-cells, natural killer cells etc., is known as cell mediated immunity. It is effective against both exogenous and endogenous antigens.
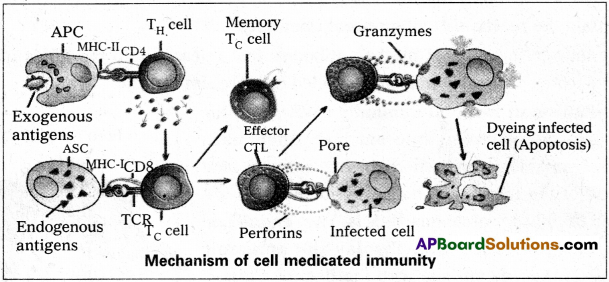
Mechanism of cell mediated immunity :
Exogenous antigens are processed by the antigen presenting cells (APC), whereas endogenous antigens are processed by altered self cells (ASCs). Then the processed antigenic fragments are displayed on their surface with the help of class-I and class-II MHC molecules of ASCs and APCs respectively They are recognised by TCR of T-cells. The binding of T-cells to APCs or ASCs cause the production of a activated T-cells and T-memory cells.
The activated TH cells secrete various types of interleukins which transform activated TC cells into effector cytotoxic T-lymphocytes. They attach to the infected or altered cells and release enzymes like perforins and granzymes. Perforins form pores in the cell membrane of the infected cells. Then granzymes enter the infected cells through these perforations and activates the proteins which help in the distinction of the infected cell by a process called apoptosis The NK cells are similar in their action to CTL’s.
![]()
Question 6.
Explain the mechanism by which HIV multiplies and leads to AIDS.
Answer:
AIDS is a non-congenital, transmissible, lethal, sexually transmitted disease caused by Human Immunodeficiency Virus (HIV). HIV is a retrovirus with an envelope enclosing two ss RNA molecules as the genetic material.
Mechanism :
After getting into the body of a person, HIV enters the TH cells, macrophages, or dendritic cells. In these cells ss RNA of HIV synthesizes a DNA strand complementary to the viral RNA using the enzyme reverse transcriptase. The same enzyme is responsible for the formation of the second DNA strand, complementary to the first strand forming the double-stranded viral DNA. This dsDNA gets incorporated into the DNA of the host’s DNA by a viral enzyme called integrase and it is in the form of a provirus.
Transcription of DNA results in the production of RNA, which can act as the genome for new viruses and can be translated into viral proteins. The various components of the viral particles are assembled and the HIV particles are produced. The infected human cells continue to produce virus particles. New viruses bud off from the host cell and attack other TH cells. This leads to decrease CD4 receptors containing TH cells in the infected person leading to immunodeficiency in him, finally causing AIDS.