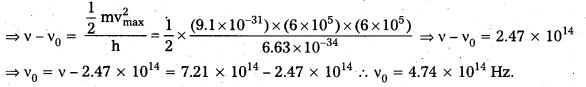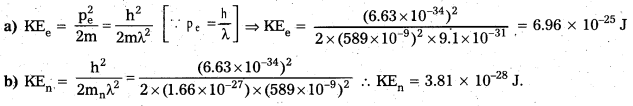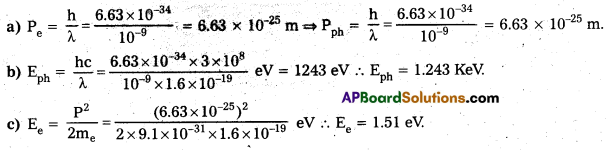Andhra Pradesh BIEAP AP Inter 2nd Year Civics Study Material 7th Lesson State Legislature Textbook Questions and Answers.
AP Inter 2nd Year Civics Study Material 7th Lesson State Legislature
Long Answer Questions
Question 1.
Explain the composition, powers, and functions of the State Legislative Assembly.
Answer:
The Constitution provides for a Legislature for every State on the model of the Parliament. As per Article 168, the State Legislature consists of the Governor and one or two Houses. In India, while some States have Bicameral Legislatures, others have Unicameral Legislatures. Andhra Pradesh at present possesses Unicameral Legislature.
The Lower House of the State Legislature is known as the Legislative Assembly or Vidhana Sabha and the Upper House is the Legislative Council or Vidhana Parishad.
Composition of Legislative Council (Vidhana Parishad) :
The Upper House of the State Legislature is known as Legislative Council. The Constitution lays down that a Legislative Council shall have not less than 40 members and not more than \(\frac{1}{3}\)rd of the total membership of the State Assembly. The Legislative Council consists of both nominated and elected members.
The election is conducted through the indirect method by means of proportional representation with a single transferable vote.
Distribution of Seats :
- \(\frac{1}{3}\)rd are elected by the members of the State Assembly.
- \(\frac{1}{3}\)rd are elected by members of local bodies.
- \(\frac{1}{12}\)th are elected by teachers.
- \(\frac{1}{12}\)th are elected by graduates.
- The remaining \(\frac{1}{6}\)th members are nominated by the Governor from among persons who have distinguished themselves in the fields of Literature, Science, Arts, Social Services etc.
Qualifications :
The members of the Council 1) must be citizens of India, 2) must have completed 30 years of age and 3) must possess such other qualifications as may be prescribed by the Legislature.
Term :
the members are elected for a period of 6 years. But \(\frac{1}{3}\)rd of them retire for every 2 years. The Council is a permanent body. It cannot be dissolved by the Governor.
Chairman and Deputy Chairman:
The Council has Chairman and a Deputy Chairman who are elected by the members of the Council from among themselves. The Chairman presides over the meetings of the Council.
Legislative Assembly (Vidhana Sabha) :
Composition of the Legislative Assembly :
The Legislative Assembly is the popular and powerful chamber of the State Legislature. It is the lower house and resembles more or less the Lok Sabha at the Centre. It consists of representatives directly elected by the people of the State on the basis of universal adult franchise. It’s maximum strength is fixed at 500 and minimum strength at 60. Only the Legislative Assembly of Sikkim has less than 60 because of it’s small population. Those who become members of State Legislative Assembly must be citizens of India and must be above 25 years of age.
Term of Office :
The normal term of Assembly is 5 years. It may be dissolved earlier by the Governor on the advice of the Chief Minister. The Parliament may extend it’s term by one year, when National Emergency is in force.
Presiding Officers :
The Presiding officer of Assembly is known as Speaker. He is elected by the members of the Assembly. The Assembly also elects a Deputy Speaker to conduct the business of the House in the absence of the Speaker.
Powers and Functions of the State Legislature :
The State Legislature has the following powers.
1) Legislative Powers and Functions :
The State Legislature has the power to make laws on all the subjects included in the State List. It has also the power to make laws in respect of subjects included in the Concurrent List. However, such a law should not disagree with a law already made by the Parliament on the same subject. In the making of laws, Legislative Assembly has been given more powers than the Legislative Council. The Legislative Council at the most may delay the legislation for a period of 4 months. Later the Assembly sends the bill to the Governor for his assent.
2) Constitutional Powers and Functions :
Even though, the Legislature has no powers to move the Constitution amendment bills, it’s consent is required for amending certain provisions of the Constitution. Such bills have to be referred to it after they are approved by the Parliament.
3) Executive Powers and Functions :
The State Legislature exercises control over the Council of Ministers. It’s members make the Ministers individually and collectively responsible to the Legislature. The Council of Ministers is collectively responsible to the State Legislative Assembly. The Legislature can expose the actions of Executive, through questions, debates and adjournment motions. In controlling the Executive, the Legislative Assembly has more powers than the Council. The Ministry has to resign when the Legislative Assembly passes no confidence motion against the Government.
4) Financial Powers and Functions:
The State Legislature exercises complete control over the finances of the State Government. It sanctions money to the State Government to enable it to run the administration. It may pass, reduce or reject the demands for grants presented to it by the Government. It may accept or reject proposals for taxation and borrowings presented to it by the Government. In financial matters the Assembly is more powerful than the Council. Because all money bills, including the Budget, shall be introduced first only in the Assembly. It can accept or reject any recommendations made by the Council.
5) Electoral Powers :
The elected members of the Assembly participate in the election of the President. They also elect the representatives of the State to the Rajya Sabha and l/3rd members of the Legislative Council if the State Legislature is bicameral. They also elect the presiding officers and deputy presiding officers of Assembly and Council.
Miscellaneous Powers : The state legislature :
- Safeguards the dignity and privileges of its members.
- Suspends, expels or terminates their membership.
- Examines the report of the State Public Service Commission and the Comptroller and Auditor General etc.
Conclusion:
The State Legislature plays an important role in the State Administration. It makes necessary laws for the welfare of the people of the State. It controls the Executive by making it responsible for their actions.
![]()
Question 2.
Write briefly the composition, powers and functions of the. State Legislative Council.
Answer:
The Upper House of the State Legislature is known as Legislative Council. The Constitution lays down that a Legislative Council shall have not less than 40 members and not more than \(\frac{1}{3}\)rd of the total membership of the State Assembly. The Legislative Council is a body of partly nominated and partly elected members.
The election is conducted through indirect method by means of proportional representation with a single transferable vote.
Composition of the Council:
- \(\frac{1}{3}\)rd members are elected by the Legislative Assembly.
- \(\frac{1}{3}\)rd members are elected by members of local bodies.
- \(\frac{1}{12}\)th members are elected by teachers.
- \(\frac{1}{12}\)th members are elected by graduates.
- The remaining \(\frac{1}{6}\)rd members are nominated by the Governor from among persons who have distinguished themselves in the fields of Literature, Science, Arts, Social Services etc.
Tenure :
The Legislative council is a quasipermanent House. l/3rd of the members of this House retire every two years. But the term of each member is six years. New members are elected in the place of retired members. All the members of the House do not retire at a time as it is a permanent House.
Powers and Functions of State Legislative council:
State Legislative council is the primary law making body along with the Legislative Assembly. The State Legislative Council has the following powers and Functions.
a) Legislative powers and Functions :
The Legislative Council does not possess equal powers and functions when compared to counterpart, State Legislative Assembly. It is said that the Legislative Council enjoys equal status and not power. However it exercises the following powers and functions. All the bills, other than money bills may be introduced in either of the House. They will be sent to the assent of the Governor only with the approval of both the Houses. The Council may reject any bill and spnt it back for the reconsideration of the Assembly. However, incase of a disagreement between two Houses, the decision of the Assembly will be supreme.
The Council must approve all the bills sent by the Assembly with in a period of three months or at the maximum of four months. It implies that the Council can withhold its assent over the bills sent by the Assembly for a maximum period of four months. Thus, the Legislative Council can only delay the bills but the Legislative Assembly can override it.
b) Executive Powers and Functions :
The State Legislative Council has very limited executive powers when compared to that of the Assembly. The council of Ministers headed by the Chief Minister is responsible for its acts only to the Assembly and not to the Council. The Council cannot decide the future of the Council of Ministers. However, the Council can influence the policies and programmes of the ministers by asking questions and supplementary questions by drawing the call attention motion etc., but they cannot force the Council of Ministers to resign.
c) Financial Powers and Functions :
The Legislative Council has only limited powers in the financial matters. Money bills cannot at first be introduced in the Legislative Council first. The Council must accept all money bills with or without recommendation within fourteen days of the receipt of the bill. The Assembly possess the discretion powers either to accept or reject these recommendations. If the Council does not return the Money Bill to the Assembly within 14 days, then the bill is deemed to have been passed by both the Houses. It is clear that in the financial field the Legislative Council has a subordinate status and that Legislative Assembly has dominant position.
d) Electoral Functions:
The Legislative Council elects a Chairman and Deputy Chairman to preside over its meetings in a dignified manner. Some of its members are elected to various legislative committees like Public Accounts Committee, Estimates Committee and Public Undertakings committee etc.
e) Other Functions :
The Legislative council acts as the best means for formulating and consolidating public opinion. It discusses technical and other contemporary matters, as there are experts in various fields.
Question 3.
Explain the role and responsibilities of the Speaker of Legislative Assembly.
Answer:
The members of State Legislative Assembly elect one among them as a speaker to conduct the business of the House. His term of office is five years.
The speaker is the guardian of the Rights and Liberties of the members of the House.
Role and Responsibilities (or) Powers and Functions of the Speaker :
The powers and functions of the speaker of State Legislative Assembly are almost the same as those of the Speaker of Lok Sabha. His powers and functions are as follows.
- The speaker preserves order and decorum in the House for conducting legislative business.
- He allocates time for different kinds of business in the House.
- He interprets the rules and procedure.
- He puts matters to vote and announces the results.
- He has the right of casting vote in case of a tie.
- He admits motions, resolutions and points of order.
- He is empowered to adjourn the meeting of the House in the absence of a quorum.
- He can order for removal of indecent and incriminatory references from the records.
- He allows the members to speak in the House.
- He may name a member and ask him to leave the House in case of disorderly behavior.
- He can adjourn the House in case of grave disorder or serious matter.
- He accepts and rejects the resignation of a member of the House after ascertaining whether it was submitted under due process or not.
- He appoints the Chairmen of all the committee of the assembly and supervises their functioning. He himself is a Chairman of Business Advisory Committee, Rules Committee and the General Purpose Committee.
- He decides where a bill is a Money Bill or not. His decision on this question is final.
Short Answer Questions
Question 1.
Write a note on the Legislative Assembly.
Answer:
Legislative Assembly is the Lower House of the State Legislature. The Members of Legislative Assembly are called M.L.As. According to Article 170of the Indian Constitution it consists of not more than 500 members and not less than 60 members. It means that it’s strength depends on the population and size of the state. But small states have been allowed to have less number of members. Thus Goa and Mizoram have only 40 members, while Sikkim has 32 Members.
Composition of the Legislative Assembly:
The Legislative Assembly is the popular and powerful chamber of the State Legislature. It is the lower house and resembles more or less the Lok Sabha at the Centre. It consists of representatives directly elected by the people of the State on the basis of universal adult franchise. Those who become members of State Legislative Assembly must be citizens of India and must be above 25 years of age.
Term of office :
The normal term of Assembly is 5 years. It may be dissolved earlier by the Governor on the advice of the Chief Minister. The Parliament may extend it’s term by one year, when National Emergency is in force.
Presiding officers :
The Presiding Officer of Assembly is known as Speaker. He is elected by the members of the Assembly. The Assembly also elects a Deputy Speaker to conduct the business of the House in the absence of the speaker.
![]()
Question 2.
Write a note on Estimates Committee.
Answer:
According to the Rules of Procedure and Conduct of Business in the State Legislature, the Estimates committee consists of 20 members. Among them 15 members belong to Assembly. The remaining 5 members belong to Legislative Council. The members hold office for a period of one year. They are elected through in indirect election.
Functions :
The functions of the Estimates Committee in the State Legislature are the same as that of Estimates Committee of Lok Sabha. These are given here under.
- Estimates Committee exercises control over public expenditure.
- It suggests fiscal reforms in organization, the efficiency or administration reforms consistent with the policy underlying estimates.
- It advises alternative policies for securing efficiency and economy in administration.
- It examines whether the money is well laid out within the limits of the policy implied in the estimates.
- It also suggests the form in which the estimates shall be presented to the Assembly.
Question 3.
What do you know about Public Accounts Committee? [Mar.-18, 17]
Answer:
Public Accounts Committee consists of 20 members out of which 15 members belong to Assembly and 5 members belong to Legislative Council. They are elected through indirect election by following the principle of proportional representation Jor a period of one year. The Chairman is normally the member of Opposition Party. The Ministers of Cabinet cannot be member of Public Accounts Committee.
Functions :
Public Account Committee performs the following functions.
- The committee examines the accounts showing the appropriation of sums granted by the house for expenditure of the state government.
- It scrutinizes the appropriation accounts of the state and the reports of the Comptroller and Auditor General.
- It shall be the duty of the Public Accounts Committee to examine such a trading, manufacturing, and profit and loss accounts and balance sheets and the accounts of the state government and also to consider the report of the Comptroller and Auditor General.
- The committee carefully considers the accounting and audit procedures.
- The committee is not concerned with the question of policy approved by the legislature.
- The committee investigates expenditure after it has already incurred. An overall, this committee is generally described as a ‘post-mortem committee’.
Question 4.
Write the powers and functions of Vidhcma Sabha Speaker. [Mar. 16]
Answer:
The powers and functions of the Speaker of State Legislative Assembly are almost the same as those of the Speaker of Lok Sabha. His powers and functions are as follows.
- The speaker preserves order and decorum in the House for conducting legislative business.
- He allocates time for different kinds of business in the House.
- He interprets the rules and procedure.
- He puts matters to vote and announces the results.
- He has the right of easting vote in case of a tie.
- He admits motions, resolutions and points of order.
- He is empowered to adjourn the meeting of the House in the absence of a quorum.
- He can order for removal of indecent arid incriminatory references from the records.
- He allows the members to speak in the House. ‘
- He may name a member and ask him to leave the House in case of disorderly behavior.
- He can adjourn the House in case of grave disorder or serious matter.
- He accepts and rejects the resignation of a member of the House after ascertaining whether it was submitted under due process or not.
- He appoints the Chairmen of all the committees of the assembly and supervises their functioning. He himself is a Chairman of Business Advisory Committee, Rules Committee and the General Purpose Committee.
- He decides where a bill is a Money Bill or not. His decision on this question is final.
Very Short Answer Questions
Question 1.
Qualifications of M.L.A. [Mar. 18, 16]
Answer:
A person who wishes to contest for the membership of the State Legislative Assembly must be possess the following qualifications.
- He should be a citizen of India.
- He should have completed the age of 25 years.
- He should possess such other qualifications as prescribed by any act of Parliament.
- However, no person can simultaneously be a member of any House of the Parliament and of a State Legislature.
Question 2.
Qualifications of M.L.C.
Answer:
A person who wishes to contest for the membership of the State Legislative Council must possess the following qualifications.
a) He should be a citizen of India.
b) He should have completed 30 years of age.
c) He should possess such other qualifications as laid down by an Act of Parliament.
Question 3.
Quorum.
Answer:
Quorum is the minimum number of members required to be present in the house before it can transact any business. According to Article 188 of the constitution, the Quorum for conducting,the State Legislative Assembly meeting was fixed at 1/10th of the total membership. However, in some states, where the strength of the State Legislative Assembly is very less, the quorum will be a minimum number of 10. The speaker decides whether there is a quorum or not on a particular day.
Question 4.
Salaries and Allowances of M.L.A.
Answer:
The salary of MLA is decided by the respective State Legislature as per the Article 164 of the Indian constitution. The members of Andhra Pradesh State Legislative Assembly receive a monthly salary of ₹ 90,000/- which includes a basic pay of ₹ 15,000/- and constituency allowance of ₹ 75,000/-. Those legislators who are not provided government accommodation will get an additional ₹ 10,000/- as H.R.A members also get daily allowance of ₹ 800/- when the state legislature is in session.
Question 5.
Privileges of State Legislature.
Answer:
Privileges of a State Legislature are a sum of special rights, immunities and exemptions enjoyed by the State Legislatures. They are necessary in order to secure independence and effectiveness of their actions. The Houses cannot maintain the authority, dignity and honour without these privileges. They can protect their members from any obstructions in the discharge of their legislative responsibilities.
- Collective privileges
- Individual privileges
i) Collective Privileges :
The legislature has the right to publish its reports, debates and proceedings and also to prohibit others publishing the same.
ii) Individual Privileges:
The privileges belonging to the members of state legislature individually. They can not be arrested during the session of the state legislature or 40 days before and after the end of the session.
![]()
Question 6.
Brief History of AP legislature.
Answer:
The Andhra state was formed on October 1, 1953 Andhra State legislature initially had 140 MLAs. Elections were held to the Andhra State Legislative Assembly for the first time in 1955.
As per the recommendations of states re-organization committee, Hyderabad State was merged with Andhra State on linguistic basic and formed into Andhra Predesh State which had 245 MLAs [Including 150 MLAs of Hyderabad State.] Elections were held to the Andhra Pradesh legislative Assembly in 1957.
The State Legislative Council was established on July 1,1958. Since then it continued to exist till June 1, 1985, before being abolished. Again on March 30, 2007 the Andhra Pradesh Legislature became again bicameral after the revival of the legislative council.
Question 7.
Chairman of Legislative council. [Mar. 16]
Answer:
There will be a chairman in the Legislative council for conducting the meetings. He is elected by the members of the Legislative council among themselves. Dr. A. Chakrapani Yadav is the Present Chairman of Legislative council of Andhra Pradesh.
Question 8.
Deputy Speaker.
Answer:
The members of State Legislative Assembly elect one among them as a Deputy Speaker. His term of office is 5 years. The deputy speaker performs the duties in the absence of the speaker.
Question 9.
Deputy Chairman of Legislative Council.
Answer:
The Members of State Legislative Council elect one among them as a Deputy Chairman. The Deputy Chairman performs the duties in the absence of the Chairman.
![]()
Question 10.
Types of committees. [Mar. 18]
Answer:
The committees are of two types i.e., Standing committees and Ad-hoc committees.
i) Standing Committees:
Standing committees deal with specific business (financial matters) Ex : Estimates committee, Public accounts committee and Committee on public undertakings.
ii) Ad-hoc Committees :
Ad-hoc committees are concerned with the matters of temporary nature. They cease to exist after completion of the work. They perform some specific functions assigned to them from time to time.