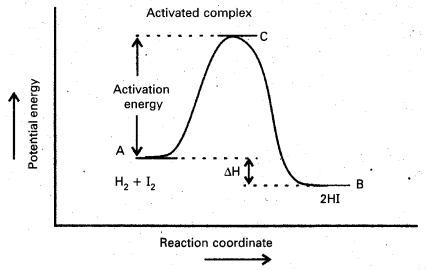
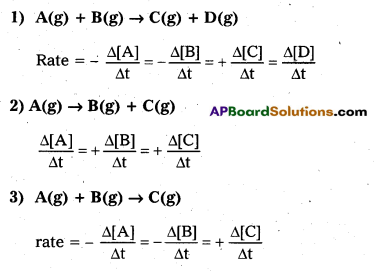
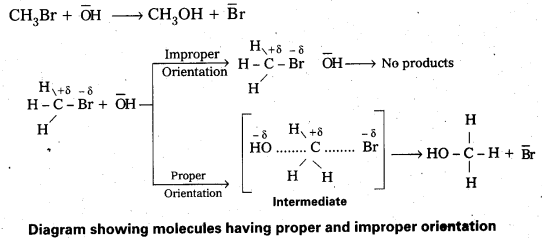
Andhra Pradesh BIEAP AP Inter 2nd Year Chemistry Study Material Lesson 6(d) Group-18 Elements Textbook Questions and Answers.
AP Inter 2nd Year Chemistry Study Material Lesson 6(d) Group-18 Elements
Very Short Answer Questions
Question 1.
What inspired Bartlett for carrying out reaction between Xe and PtF6 ?
Answer:
- At first Bartlett prepared a red compound [latex]\mathrm{O}_2^{+}+\mathrm{PtF}_6^{-}[/latex]
- As the first Ionisation Enthalpy of molecular oxygen is identical with that the Xe.
- He made efforts to prepare same type of compound with Xe and was successful in preparing another red colour compound Xe+ [latex]\mathrm{PtF}_6^{-}[/latex] by mixing PtF6 and Xe.
Question 2.
Which of the following does not exist ?
a) XeOF4
b) NeF2
c) XeF2
d) XeF6
Answer:
Given compounds are XeOF4, NeF2, XeF2, XeF6.
NeF2 does not exist among the above compounds.
Reason : Due to small size and high I.E. of ‘Ne’. It does not form chemical compounds.
![]()
Question 3.
Why do noble gases have comparatively large atomic sizes ?
Answer:
Noble gases have comparatively large atomic sizes as they have vander waals radii which is larger than both the ionic and covalent radii.
Question 4.
List out the uses of Neon. [A.P. Mar. 18]
Answer:
Uses of Ne:
- Ne is used in discharge tubes and fluor escent bulbs for advertisement display purpose.
- ‘Ne’ – bulbs are used in botanical gardens and in green houses.
Question 5.
Write any two uses of argon.
Answer:
Uses of Ar :
- ‘Ar’ is used to create inert atmosphere in high temperature net allurgical process.
- ‘Ar’ is ued in filling electric bulbs.
Question 6.
In modern diving apparatus, a mixture of He and O2 is used – Why ? [A.P. Mar. 16]
Answer:
In modem diving apparatus, a mixture of He and O2 is used because He is very low soluble in blood.
Question 7.
Helium is heavier than hydrogen. Yet helium is used (instead of H2) in filling baloons for meteorological observations – Why ?
Answer:
‘He’ is a non-inflammable and light gas. Hence it is used in filling baloons for meterological observations.
![]()
Question 8.
How is XeO3 prepared ?
Answer:
XeF6 on hydrolysis produce XeO3
XeF6 + 3H2O → XeO3 + 6HF
Question 9.
Give the preparation of
a) XeOF4 and
b) XeOaF2
Answer:
Partial hydrolysis of XeF6 gives oxy fluorides XeOF4 and XeO2F2
XeF6 + H2O → XeOF4 + 2HF
XeF6 + 2H2O → XeO2F2 + 4HF
Question 10.
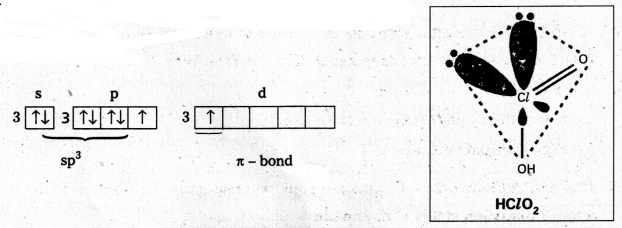
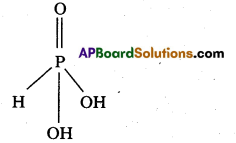
Explain the structure of XeO3. [T.S. Mar. 16]
Answer:
Structure of XeO3
- Central atom is ‘Xe’
- ‘Xe’ under goes sp3 hybridisation in 3rd excited state.
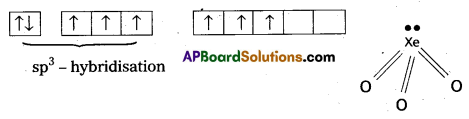
- ‘Xe’ forms 3σ-bonds and 3π-bonds with three oxygens.
- Shape of molecule is pyramidal with bond angle 103°.
Question 11.
Noble gases are inert – explain.
Answer:
Noble gases are chemically inert:
- Noble gases have stable electronic configuration coctet configuration except He)
- Noble gas have high Ionisation energy values and have large positive values of electron gain Enthalpy.
Question 12.
Write the name and formula of the first noble gas compound prepared by Bertlett.
Answer:
The first noble gas compound prepared by Bertlett is XePtF6. Name of the compound is xenon hexa fluoro platinate.
![]()
Question 13.
ExplaIn the shape of XeF4 on the basis of VSEPR theory
Answer:
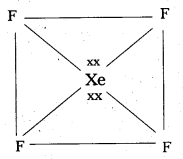
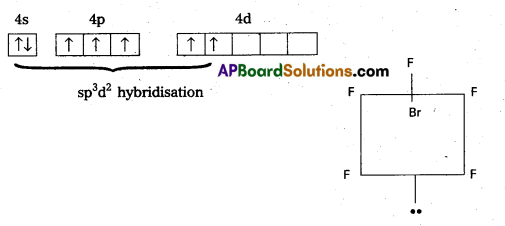
Shape of XeF4:
- Central atom in XeF4 is ‘Xe’.
- Xe undergoes sp3d2 hybridisation in it’s 2nd excited. state.
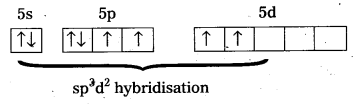
- Shape of the molecule is square planar with bond angle 90° and bond length 1 .95Å.
- Xe – forms four o-bonds by the overlap of sp3d2 – 2pz(F) orbitals.
Question 14.
Give the outer electronic configuration of noble gases.
Answer:
The outer electronic configuration of noble gases is ns2np6 (except He (1s2)
Question 15.
Why do noble gases form compounds with fluorine and oxygen only ?
Answer:
Noble gases form compounds with flourine and oxygen only.
Reason : Oxygen and Fluorine are most electronegative elements.
Question 16.
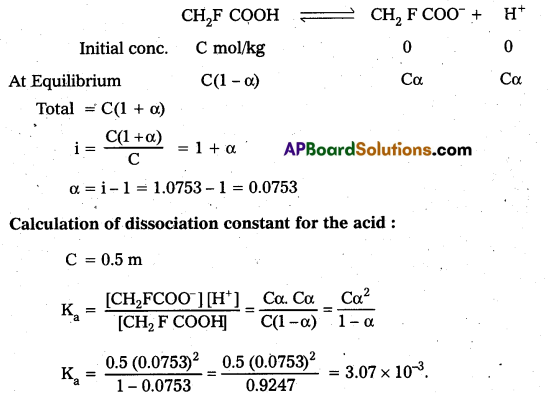
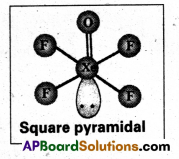
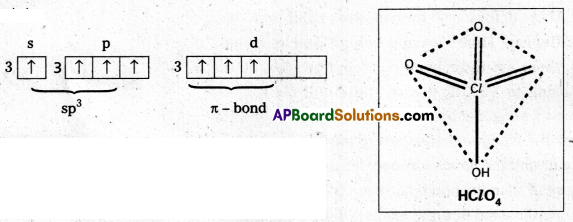
How is XeOF4 prepared ? Describe its molecular shape.
Answer:
Partial hydrolysis of XeF6 gives XeOF4
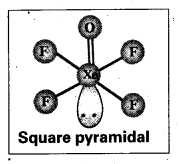
XeF6 + H2O → XeOF4 + 2HF
XeOF4 is a colourless volatile liquid it has a square pyramidal shape.
![]()
Question 17.
What is the major source of helium ?
Answer:
The major source of helium is natural gas.
Question 18.
Which noble gas is radioactive ? How is it formed ?
Answer:
Radon (Rn) is radio active noble gas. Radon is obtained as decay product of 86Ra226
86Ra226 → 86Rn222 + 2He4
Question 19.
Name the following :
a) most abundant noble gas in atmosphere
b) radioactive noble gas
c) noble gas with least boiling point
d) noble gas forming large number of compounds
e) noble gas not present in atmosphere
Answer:
a) Argon is the most abundant noble gas in atmosphere.
b) Radon is the radio active noble gas.
c) Helium has lowest boiling point (4.2K)
d) Xenon forms large number of compounds.
e) Radon is not present in atmosphere.
Short Answer Questions
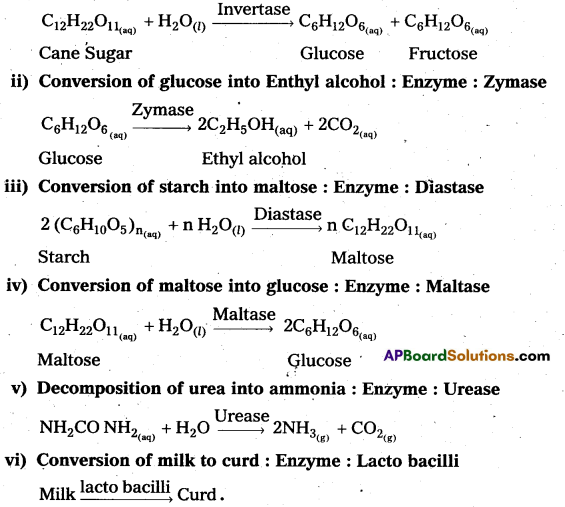
Question 1.
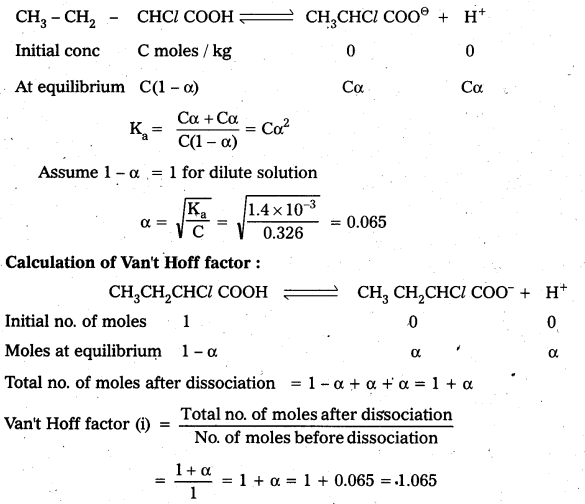
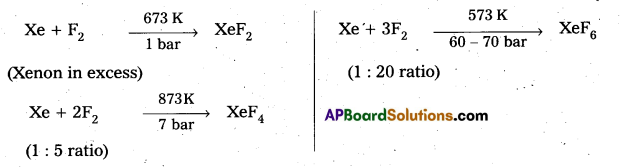
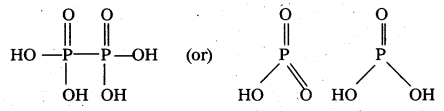
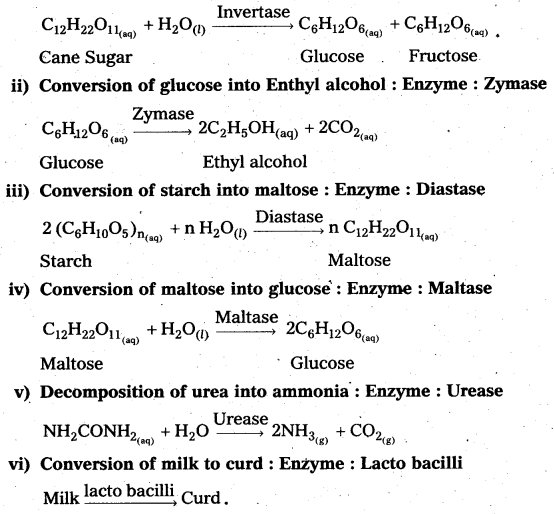
How are Xenon fluorides XeF2, XeF4 and XeF6 obtained?
Answer:
Xenon forms the binary fluorides XeF2, XeF4, XeF6 as follows. These are formed by direct combination of Xe and F2.
Question 2.
How are XeO3 and XeOF4 prepared? [Mar. 14]
Answer:
XeF6 on hydrolysis produce XeO3
XeF6 + 3H2O → XeO3 + 6HF
Partial hydrolysis of XeF6 gives XeOF4
XeF6 + H2O → XeOF4 + 2HF
Question 3.
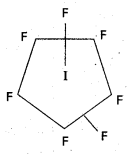
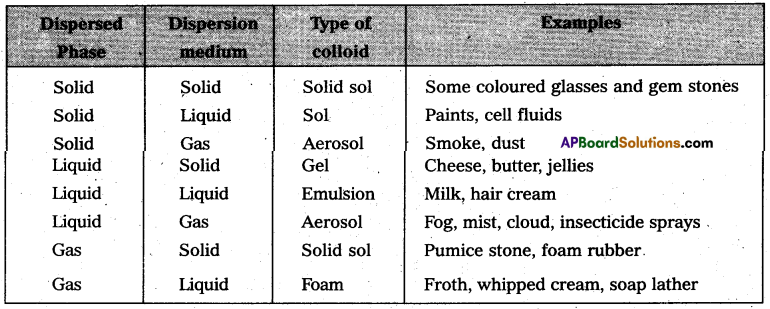
Give the formulae and describe the structures of a noble gas species, isoelectronic with
a) ICl4
b) IBr2
c) BrO–3
Answer:
a) ICl4 is also electronic with XeF4 and it has square planar shape.
b) IBr–2 is also electronic with XeF2 and it has linear shape.
c) BrO–3 is also electronic with XeO4 and it has tetrahedral shape.
![]()
Question 4.
Explain the reaction of the following with water.
a) XeF2
b) XeF4
c) XeF6
Answer:
a) XeF2 is hydrolysed to form Xe, HF and O2
2XeF2 + 2H2O → 2Xe + 4HF + O2
b) XeF4 is hydrolysed to give XeO3
6XeF4 + 12H2O → 4Xe + 2XeO3 + 24 HF + 3O2
c) XeF6 undergo hydrolysis to form XeO3
XeF6 + 3H2O → XeO3 + 6HF
XeF6 undergo partial hydrolysis to form XeOF4 + XeO2F2
XeF6 + H2O → XeOF4 + 2HF
XeF6 + 2H2O → XeO2F2 + 4HF
Question 5.
Explain the structures of [A.P. Mar. 18] [Mar. 14]
a) XeF2 and
b) XeF4
Answer:
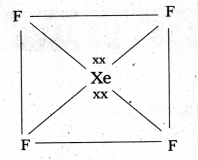
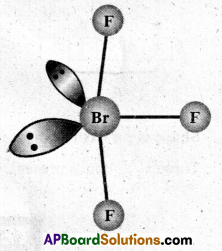
a) Structure of XeF2 :
- In XeF2 central atom is ‘Xe’.
- ‘Xe’ undergoes sp3d hybridisation in it’s 1st excited state
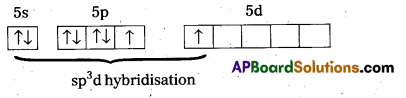
- Shape of molecule is linear
- Xe form two σ – bonds with two fluorines (sp3 – 2pz overlap)
b) Structure of XeF4 :
- Central atom in XeF4 is ‘Xe’.
- Xe undergoes sp3d2 hybridisation in it’s 2nd excited, state.
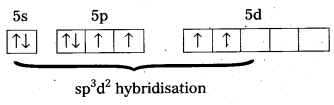
- Shape of the molecule is square planar with bond angle 90° and bond length 1.95Å.
- Xe – forms four a – bonds by the overlap of sp3d2 – 2pz(F) orbitals.
Question 6.
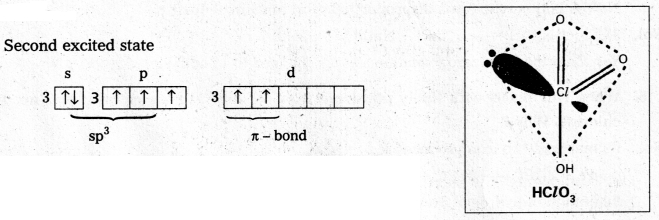
Explain the structures of
a) XeF6 and
b) XeOF4
Answer:
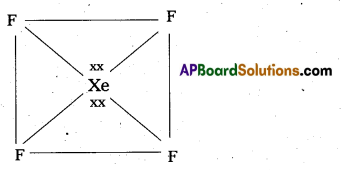
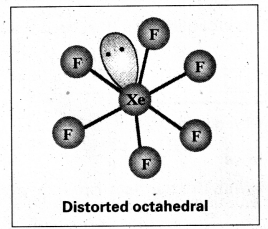
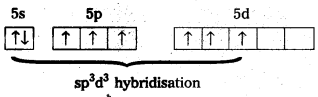
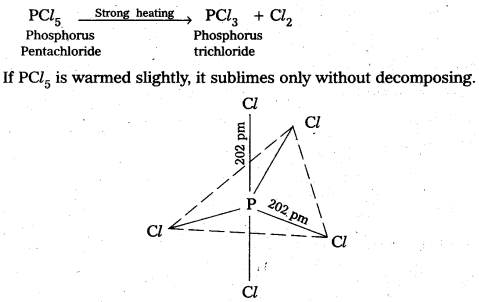
a) Structure of XeF6 :
- Central atom in XeF6 is ‘Xe’
- Xe under goes sp3d3 hybridisation in it’s 3rd excited state.
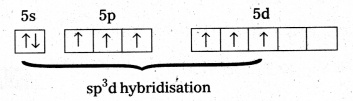
- Shape of molecule is distorted octahedral.
![]()
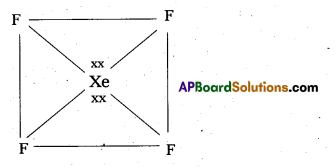
b) Structure of α XeOF4
- In XeOF4 molecule ‘Xe’ under goes sp3d2 hybridisation.
- Shape of the molecule is square pyramid.
- There is one Xe-O double bond containing
pπ = dπ overlaping.
Partial hydrolysis of XeF6 gives XeOF4
XeF6 + H2O → XeOF4 + 2HF
XeOF4 is a colourless volatile liquid it has a square pyramidal shape.
Question 7.
Complete the following.
a) XeF2 + H2O →
b) XeF2 + PF5 →
c) XeF4 + SbF5 →
d) XeF6 + ASF5 →
e) XeF4 + O2F2 →
f) NaF + XeF6 →
Answer:
a) 2XeF2 + 2H2O → 2Xe + 4HF + O2
b) XeF2 + PF5 → [XeF]+ + A[F6]–
c) XeF4 + SbF5 → [XeF3]+ [SbF6]–
d) XeF6 + ASF5 → [Xe2F11]+ [ASF6]–
e) XeF4 + O2F2 → XeF6 + O2
f) NaF + XeF6 → Na+ [XeF7]–
Question 8.
How are XeF2 and XeF4 prepared ? Give their structures. [T.S. Mar. 18]
Answer:
Xenon forms the binary fluorides XeF2, XeF4, XeF6 as follows. These are formed by direct combination of Xe and F2.

Structure of XeF2
- In XeF2 central atom is Xe’.
- ‘Xe’ undergoes sp3d hybridisation in it’s 1st excited state
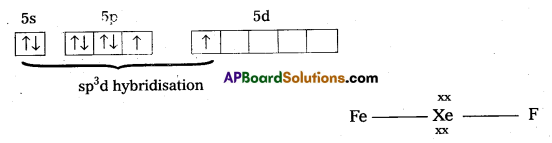
- Shape of molecule is linear.
- Xe form two σ bonds with two fluorines.
![]()
b) Structure of XeF4 :
- Central atom in XeF4 is ‘Xe’.
- Xe undergoes sp3d2 hybridisation in it’s 2nd excited state.
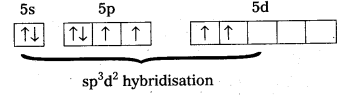
- Shape of the molecule is square planar with bond angle 90° and bond length 1.95Å.
- Xe – forms four σ-bonds by the overlap of sp3d2 – 2pz (F) orbitals.
Long Answer Question
Question 1.
How are XeF2, XeF4 and XeF6 prepared ? Explain their reaction with water. Discuss their structures. [A.P. Mar. 15]
Answer:
Xenon forms the binary fluorides XeF2, XeF4, XeF6 as follows. These are formed by direct combination of Xe and F2.
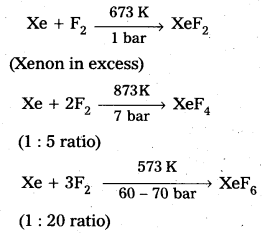
Reaction with water:
- XeF2 is hydrolysed to form Xe, HF and O2
2XeF2 + 2H2O → 2Xe + 4HF + O2 - XeF4 is hydrolysed to give XeO3
6XeF4 + 12H2O → 4Xe + 2XeO3 + 24 HF + 3O2 - XeFg undergo hydrolysis to form XeO3
XeF6 + 3H2O → XeOF3 + 6HF - XeF6 undergo partial hydrolysis to form XeOF4 and XeO2F2
XeF6 + H2O → XeOF4 + 2HF
XeF6 + 2H3O → XeO2F2 + 4HF
Structure of XeF2:
- In XeF2 central atom is ‘Xe’.
- ‘Xe’ undergoes sp3d hybridisation in it’s 1st excited state
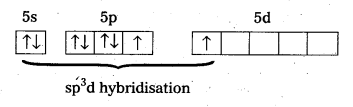
- Shape of molecule is linear.
- Xe form two σ-bonds with two fluorines.
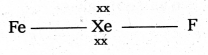
b) Structure of XeF4 :
- Central atom in XeF4 is ‘Xe’.
- Xe undergoes sp3d2 hybridisation in it’s 2nd excited state.
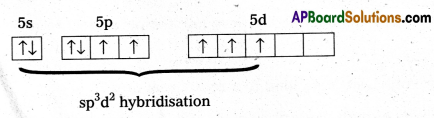
- Shape of the molecule is square planar with bond angle 90° and bond length 1.95Å.
- Xe – forms four σ-bonds by the overlap of sp3d2 – 2pz (F) orbitals.
![]()
Structure of XeF6 :
- Central atom in XeF6 is ‘Xe’
- Xe under goes sp3d3 hybridisation in it’s 3rd excited state.

- Shape of molecule is distorted octahedral.

Textual Examples
Question 1.
Why are the elements of group 18 known as noble gases ?
Solution:
The elements present in Group 18 have their valence shell orbitals completely filled and, therefore, react with a few elements only under certain conditions. Therefore, they are now known as noble gases.
Question 2.
Noble gases have very low boiling points. Why ?
Solution:
Noble gases being monoatomic have no interatomic forces except weak dispersion forces and therefore, they are liquefied at very low temperatures. Hence, they have low boiling points.
![]()
Question 3.
Does the hydrolysis of XeF6 lead to a redox reaction ?
Solution:
No, the products of hydrolysis are XeOF4 and XeO2F2 where the oxidation states of all the elements remain the same as it was in the reacting state.
Intext Questions
Question 1.
Why is helium used in diving apparatus ?
Solution:
Helium is used in diving apparatus due to its very low solubility in blood.
Question 2.
Balance the following equation :
XeF6 + H2O → XeO2F2 + HF
Solution:
XeF6 + 2H2O → XeO2F2 + 4HF
Question 3.
Why has it been difficult to study the chemistry of radon ?
Solution:
Radon is a radioactive element with very short half-life of 3.82 days. That is why, the study of chemistry of radon is a difficult task.



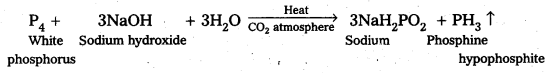

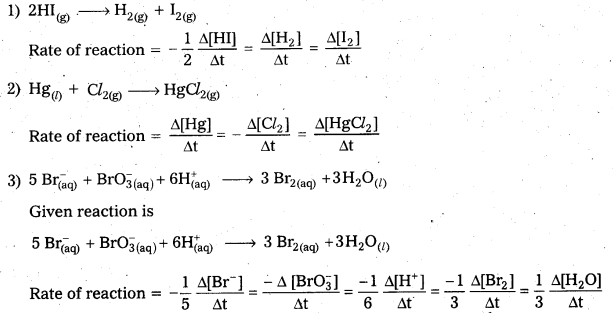


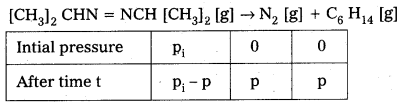
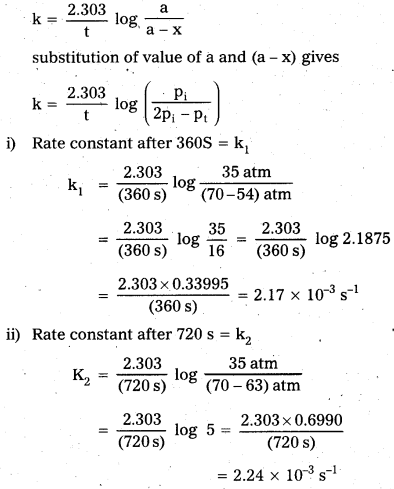
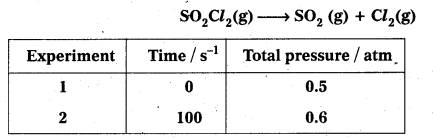

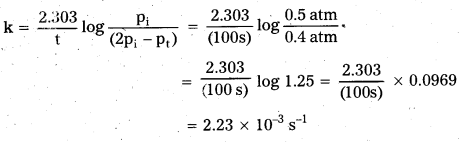
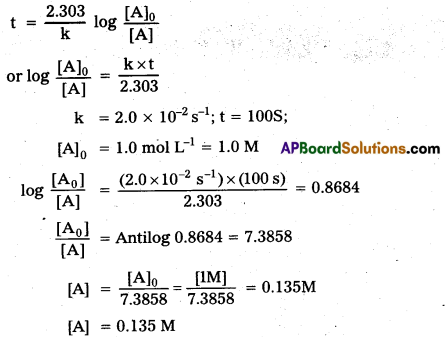
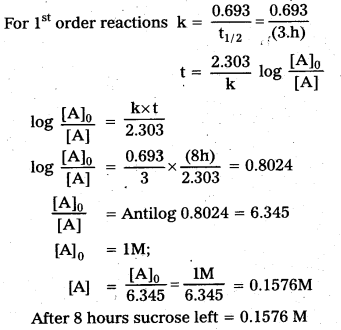
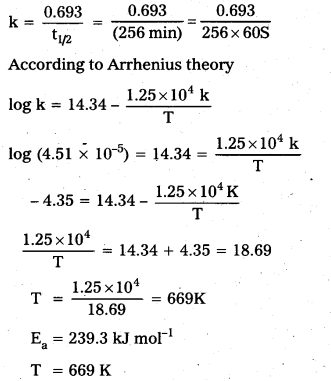
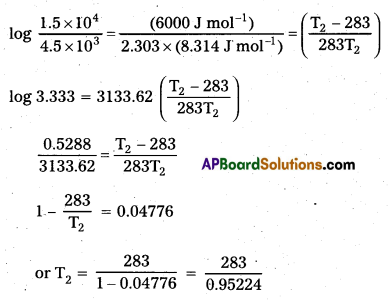
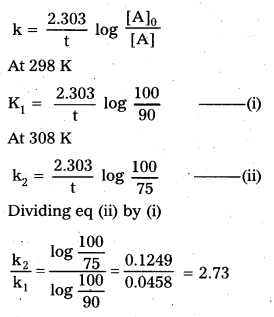
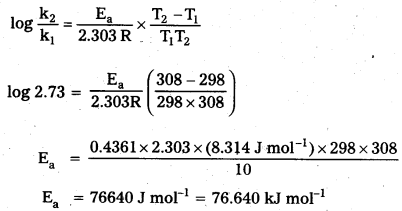
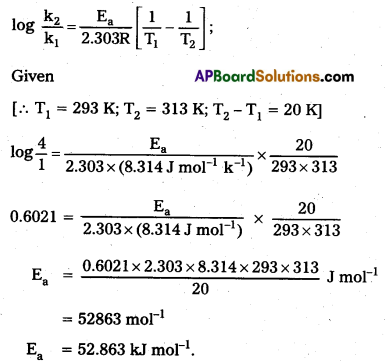
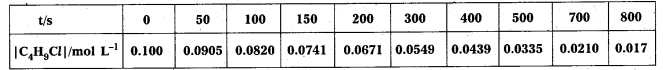

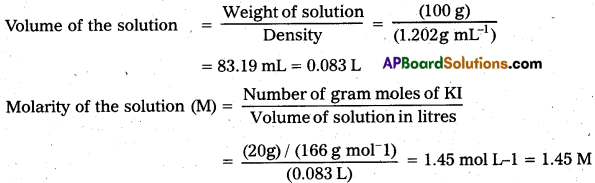
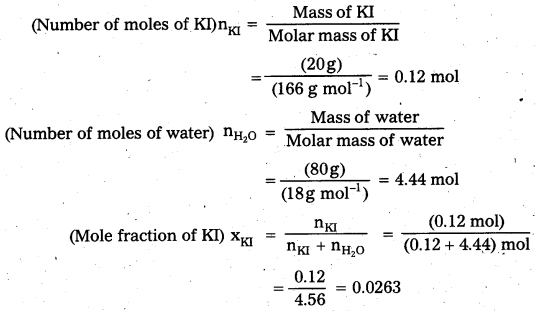
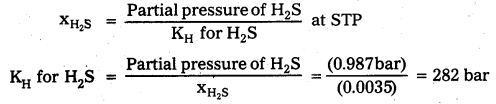
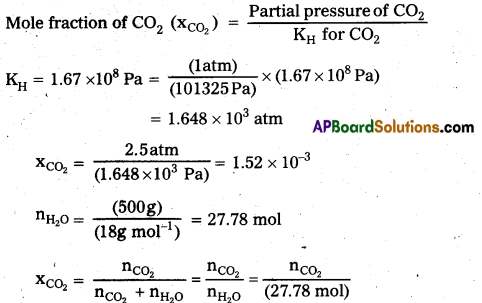
 × 100
× 100 × 106
× 106
 × 10
× 10 × 100
× 100