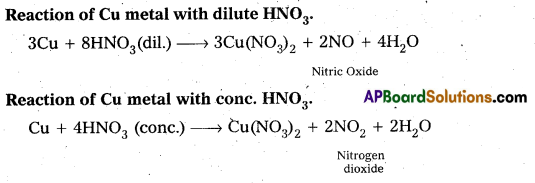
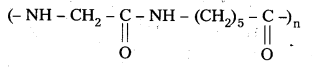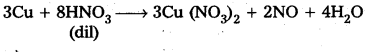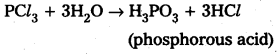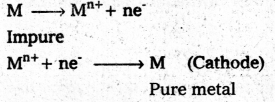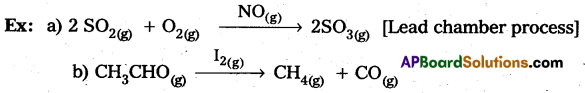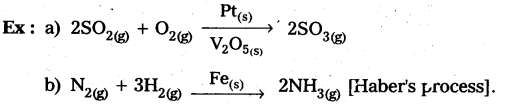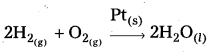Students get through AP Inter 2nd Year Chemistry Important Questions Lesson 12(b) Aldehydes, Ketones, and Carboxylic Acids which are most likely to be asked in the exam.
AP Inter 2nd Year Chemistry Important Questions Lesson 12(b) Aldehydes, Ketones, and Carboxylic Acids
Very Short Answer Questions
Question 1.
Although phenoxide ion has more resonating structures than carboxylate ion carboxylic acid is a stronger acid than phenol. Why?
Answer:
- Phenoxide ion has non-equivalent resonance structures in which the negative charge is at the less electronegative carbon atom.
- The negative charge is delocalized over two electronegative oxygen atoms in carboxylate ion whereas in phenoxide ion the negative charge is less effectively delocalized over one oxygen atom and fewer electronegative carbon atoms.

Question 2.
Write equations showing the conversion of
i) Acetic acid to Acetyl chloride
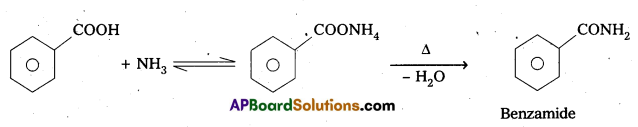
ii) Benzoic acid to Benzamide
Answer:
i) Acetic acid reacts with PCl3 (or) PCl5 (or) SOCl2 to form acetyl chloride.
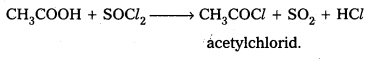
ii) Benzoic acid reacts with ammonia to form benzamide.
![]()
Question 3.
List the reagents needed to reduce carboxylic acid to alcohol.
Answer:
The reagents required to reduce carboxylic acid to alcohol are
- LiAlH4/Ether (or) B2H6
- H3O+
Question 4.
Compare the acidic strength of acetic acid, Chloroacetic acid, benzoic acid and Phenol. (IPE 2014) (Mar. ’14)
Answer:
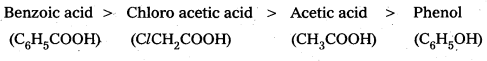
Question 5.
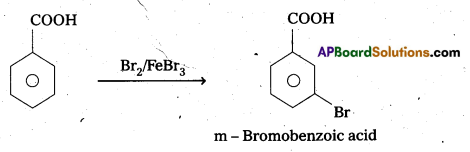
Explain the position of electrophilic substitution in benzoic acid.
Answer:
Benzoic acid undergo electrophilic substitution reactions in which carboxyl group acts as a deactivating and meta directing group.
Question 6.
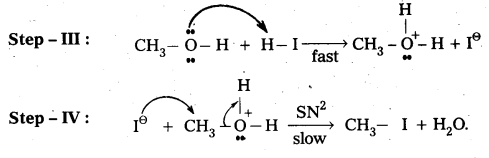
Write the mechanism of esterification.
Answer:
Mechanism of esterification of carboxylic acids : The esterification of carboxylic acids with alcohols is a kind of nucleophilic acyl substitution. Protonation of the carbonyl oxygen activates the carbonyl group towards nucleophilic addition of the alcohol. Proton transfer in the tetrahedral intermediate converts the hydroxyl group into – +OH2 group, which, being a better leaving group, is. eliminated as neutral water molecule. The protonated ester so formed finally loses a proton to give the ester.
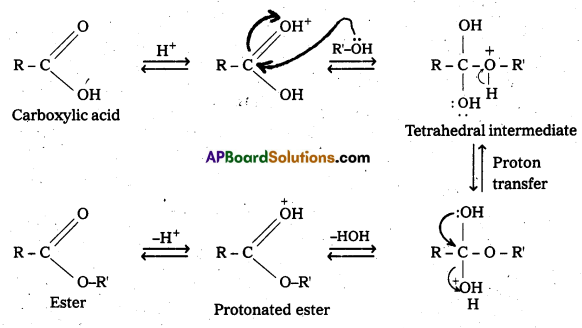
![]()
Question 7.
What is Etard reaction. Give equation.
Answer:
Etard Reaction : Benzaldehyde is prepared by the oxidation of Toluene with chromyl chloride.
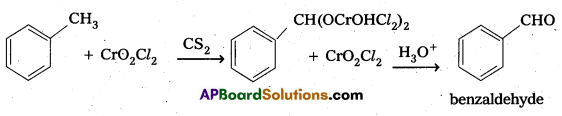
Question 8.
What is Gater man – Koch formylation reaction. Give equation.
Answer:
Gates man – Koch reaction:
Benzene or its derivatives treated with CO and HCl in presence of AlCl3 gives Benzaldehyde.
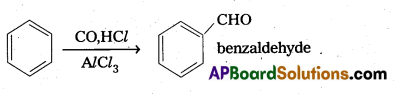
Question 9.
An organic acid with molecular formula C8H8O2 on decarboxylation forms Toluene. Identify the organic acid.
Answer:
The organic acid is phenyl acetic acid.
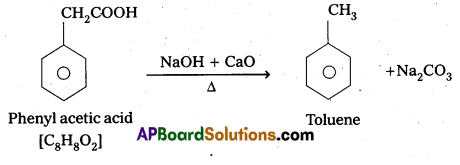
Question 10.
What is decarboxylation ? Give equation.
Answer:
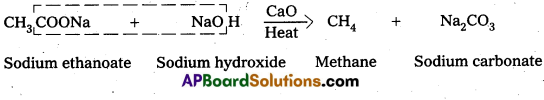
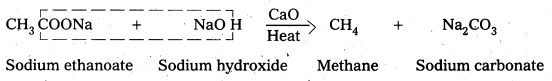
Decarboxylation: Carboxylic acids lose carbon dioxide molecule to produce hydrocarbons on heating their sodium salts with soda lime (a mixutre of NaOH & CaO In ratio 3: 1).
- This reaction is called decarboxylation.
Short Answer Questions
Question 1.
What is Tollens reagent ? Explain its reaction with Aldehydes.
Answer:
Tollens Reagent: Freshly prepared ammonical silver nitrate solution is called Tollens reagent.
- On warming an aldehyde with Tollens reagent a bright silver mirror is produced due to formation of silver metal.
R – CHO + 2 [Ag (NH3)2]+ + 3OH– → RCOO– + 2Ag + 2H2O + 4NH3
Question 2.
Describe the following:
i) Cross aldol condensation
ii) Decarboxylation
Answer:
i) Cross Aldol Condensation: When aldol condensation is carried out between two different aldehydes and (or) ketones, it is called cross aldol condensation.
- If both the reactants contain α-hydrogen atoms, it gives a mixture of four products.
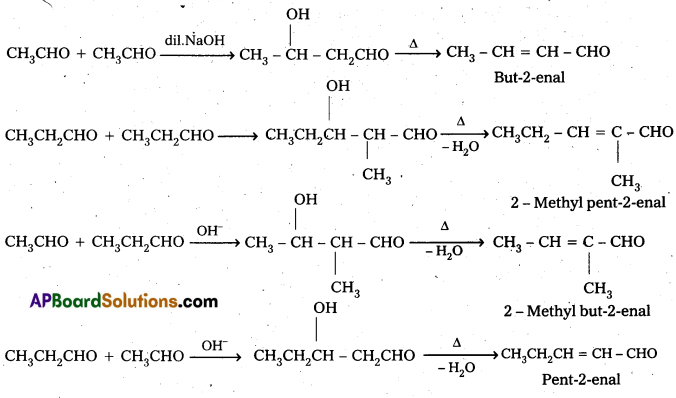
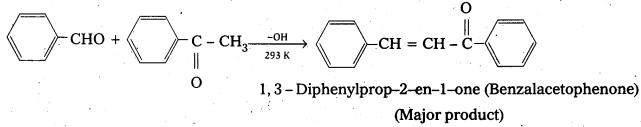
Ketones can also be used as one component in the cross aldol reactions.
ii) DecarboxylationCarboxylic acids lose carbon dioxide molecule to produce hydrocarbons on heating their sodium salts with soda lime (a mixture of NaOH & CaO in ratio 3:1).
- This reaction is called decarboxylation
![]()
Question 3.
Explain Nucleophilic addition reaction mechanism of aldehydes and ketones.
Answer:
Nucelophilic addition reaction mechanism:
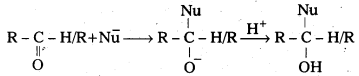
Nucleophile attack on the electrophilic carbon atom of the polar carbonyl group to form an intermediate this intermediate capture a proton to give the product. Aldehydes are more reactive towards the nucleophilic addition reaction due to steric and electronic reasons. In ketones two large substituents sterically hinder the approach of nucleophile towards carbonyl carbon. Aldehydes having only one alkyl group the attack of nucleophile is easy. Electronically two alkyl groups in ketones reduce the positive charge density on carbonyl carbon by + I effect, than aldehydes having only one alkyl group.
Question 4.
Explain the role of electron withdrawing and electron releasing groups on the acidity of carboxylic acids.
Answer:
- Electron withdrawing groups increase the acidity of carboxylic acids by stabilising the conjugate base through delocalisation of the negative charge by inductive effect.
- Electron donating groups decreases the acidity of carboxylic acids by destabilising the conjugate base.
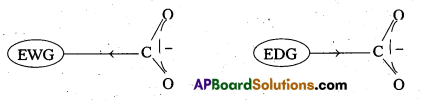
Eg : Cl– is a electron withdrawing group acidic strength order in case of chloro acetic acids CCl3COOH > CHCl2COOH > CH2ClCOOH > CH3COOH
Question 5.
Write any two methods for the preparation of Aldehydes.
Answer:
Preparation of Aldehydes:
From Hydrocarbons:
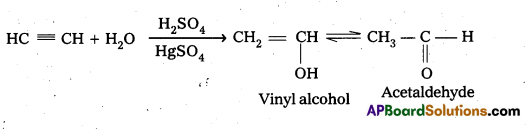
a) Hydration of alkynes in the presence of H2SO4 and HgSO4 give aldehydes and ketones.
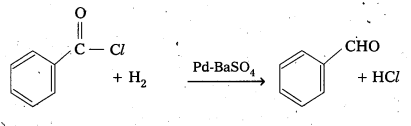
b) Reduction Acetyl Chloride (Rosen mund’s reduction)
c) From nitriles (Stephen reaction)
![]()
Nitriles are selectively reduced by di-isobutyl aluminium hydride (DIBAL – H) to imines which hydrolysis give aldehydes.
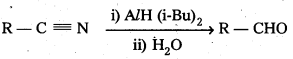
Question 6.
Write any two methods for the preparation of Ketones.
Answer:
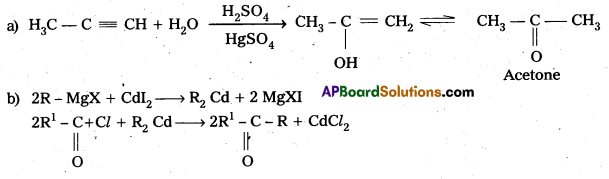
Acyl chlorides react with dialkyl cadmium give ketones.
![]()
Question 7.
Explain the following terms. Give an example of the reaction in each ease.
i) Cyanohydrin
ii) Acetal
iii) Semicarbazone
iv) Aldol
v) Hemiacetal
vi) Oxime (IPE 2016 (TS))
Answer:
i) Cyanohyclrin
Aldehydes and ketones react with hydrogen cyanide (HCN) forms addition products called (or) known as cyanohydrins.
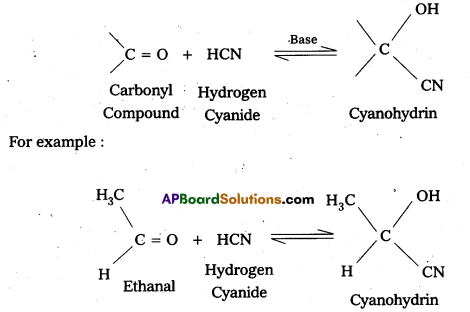
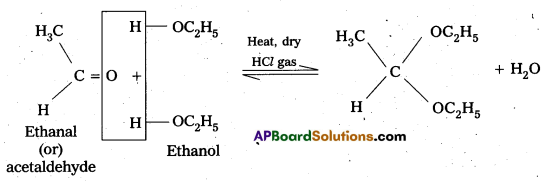
ii) Acetal
- In the presence of dry HCl gas, an aldehyde reacts with two equivalents of a monohydric alcohol forms gem-dialkoxy compounds are known as acetals.
- In acetal two alkoxy groups are present on the terminal C-atom.
iii) Semicarbazone
Aldehydes/ketones react with semicarbazide forms certain compounds called as semicarbazones.
For example:
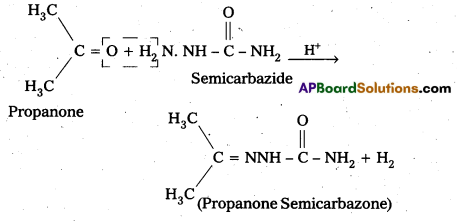
iv) Aldol
When an aldehyde ((or) ketone) having atleast one α-hydrogen atom undergo a reaction in the presence of dilute alkali as catalyst to form aldol (or) β – hydroxy aldehydes ((or) ketals in case of ketones), the reaction is called aldol condensation.
For example:
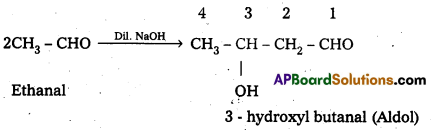
v) Hemlacetal: In the presence of dry HCl gas an aldehyde reacts with one molecule of a monohydric alcohol forms gem-alkoxy alcohols. These are known as hemiacetals.
For example:
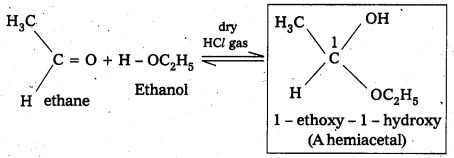
vi) Oxime : In weak acidic medium, an aldehyde ketone reacts with hydroxylamine forms products which are known as oxims.
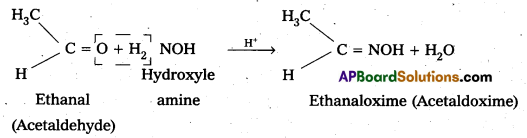
Question 8.
Explain Clemenson’s reduction and Wolf Kishner reduction reactions.
Answer:
i) Clemenson’s reduction: In this reaction carbonyl group is reduced to CH2 hence aldehydes and ketones are directly converted into alkanes. The reducing agent in this reaction is zinc amalgam and concentrated HCl.
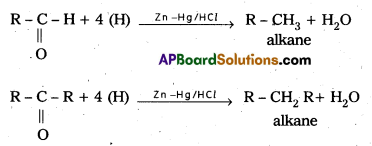
ii) Wolf Kishner reduction : In this reaction also aldehydes and ketones are reducted to alkanes. Addition of hydrazine followed by heating in the presence of KOH in glycol gives alkanes.
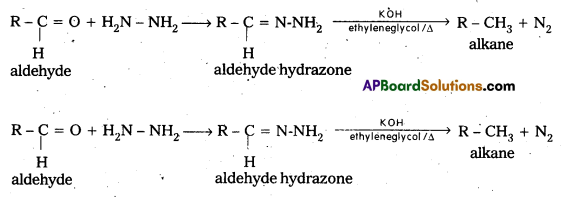
![]()
Question 9.
What is haloform reaction? Give équation.
Answer:
Haloform reaction: (Oxidation of methyl ketones)
Aldehydes and ketones having at least one (CH3CO) group react with halogens in presence of NaOH or sodium hypohalite to form haloform (CHX3) and salt of carboxylic acids having one carbon atom less than that of carbonyl compound.
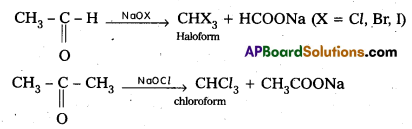
Question 10.
What is Cannizaro reaction? Give equation. (IPE – 2015 (AP), B.M.P)
Answer:
Cannizaro reaction : Aldehydes having no α – hydrogen undergo self oxidation and self reduction reaction on heating with alkali. In this reaction alcohol and salt of carboxylic acid are formed.
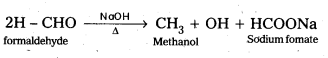
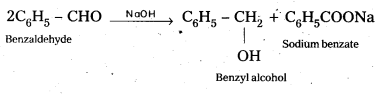
Question 11.
What is HVZ reaction? Give equation. (T.S. Mar.’18) (IPE 2016 (TS))
Answer:
Carboxylic acids having α-hydrogens are halogenated at the α-position on treatment with chlorine or bromine in presence of small amount of red phosphorus to give α-halo carboxylic acids.
This reaction is named as Hell-Volhard — Zelinsky (HVZ) reactions.
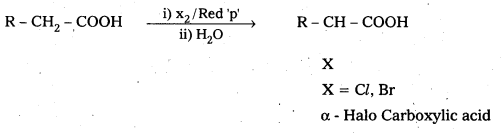
Question 12.
Write any three methods for the preparatión of Carboxylic acid.
Answer:
Preparation of carboxylic acids:
i) From primary alcohols : Primary alcohols on oxidation with acidified KMnO4 (or) K2Cr2O7 (or) Chromium trioxide (CrO3) gives carboxylic acids.
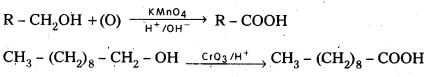
ii) From Alkyl benzenes : Aromatic carboxylic acids are prepared by the oxidation of alkyl benzenes with KMnO4 or CrO3, any alkyl group irrespective of chain length is oxidised to -COOH.
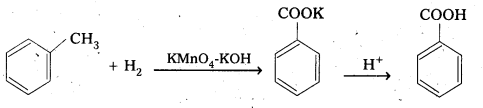
iii) From Nitrites and Amides: Nitnies on hydrolysis in the presence of H+ or OH– gives amides which on hydrolysis give acids.
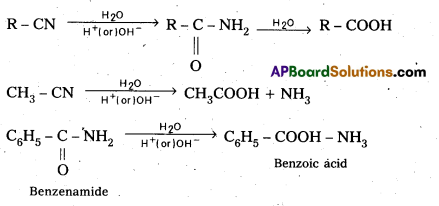
Question 13.
Explain Ring substitution reactions of aromatic carboxylic acids.
Answer:
Ring Substitution : Aromatic carboxylic acids undergo electrophilic substitution at meta position. -COOH group is electron with drawing group and deactivates the ring at ortho and para position.
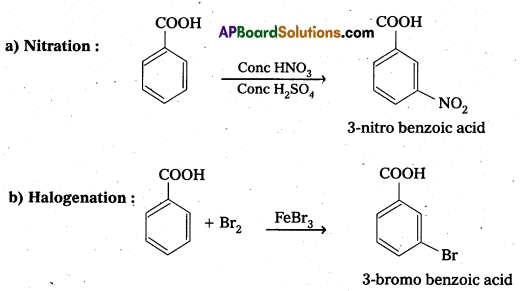
Question 14.
How do you prepare the following compounds from acetic acid?
i) Acetyl chloride
ii) Acetamide
iii) Acetic anhydride
iv) Ethyl alcohol
Answer:
i) Acetyl chloride: Reaction with PCl3 (or) PCl5 (or) SOCl2 : Carboxylic acids form acid chlorides with PCl3, PCl5, SOCl2.
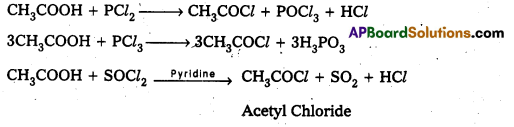
ii) Acetamide formation (Reaction with ammonia) : Carboxylic acids react with Ammonia and form ammonium salt which on heating gives amides.
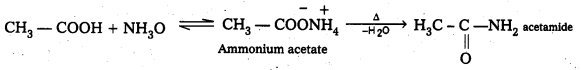
ii) Acetic anhydride : Carboxylic acids on heating in the presence of dehydrating agents like conc H2SO4, P2O5 give anhydride.
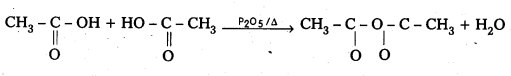
iv) Ethyl alcohol : Acetic acid is reduced to ethyl alcohol by LiAlH4.
![]()
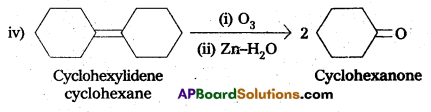
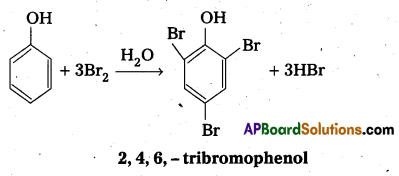
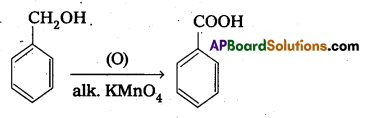
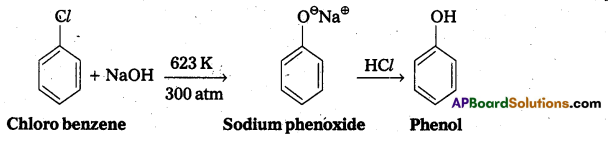
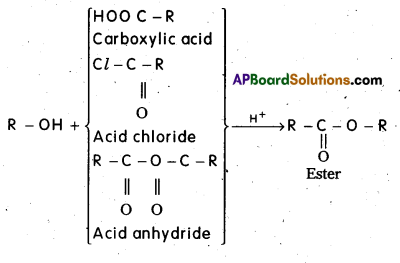
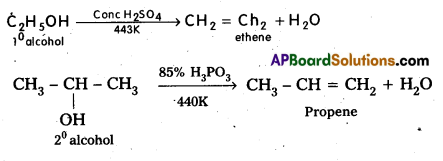
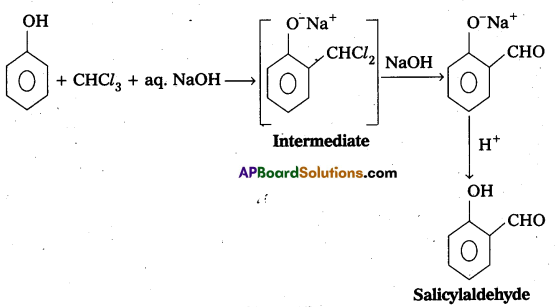
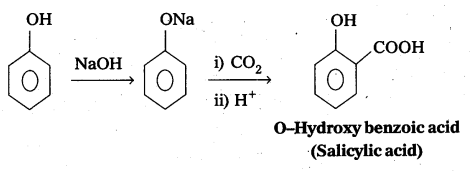
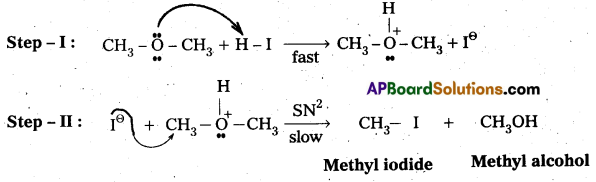
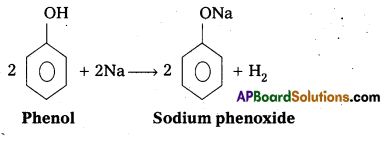
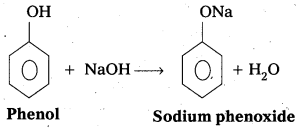
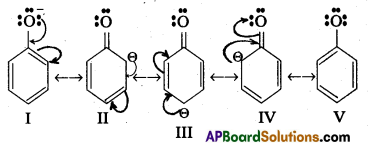
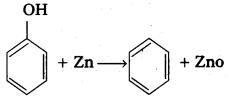
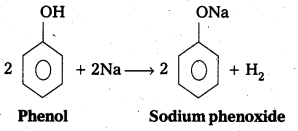
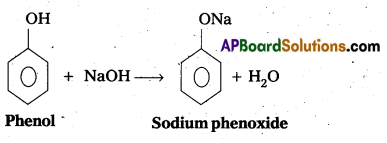
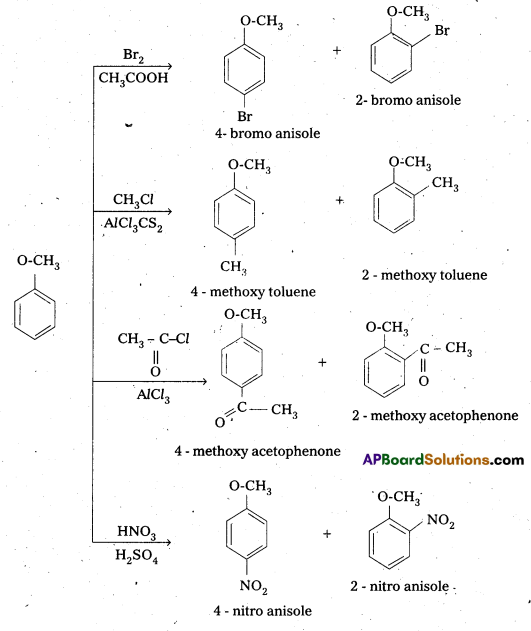
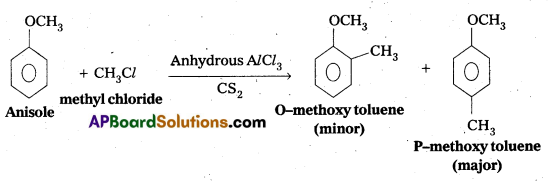
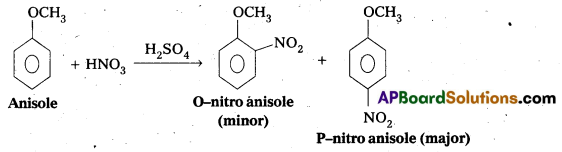
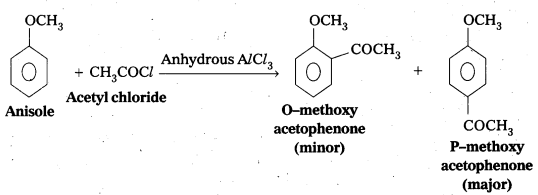
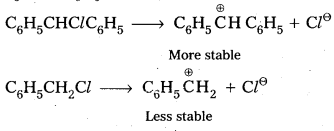

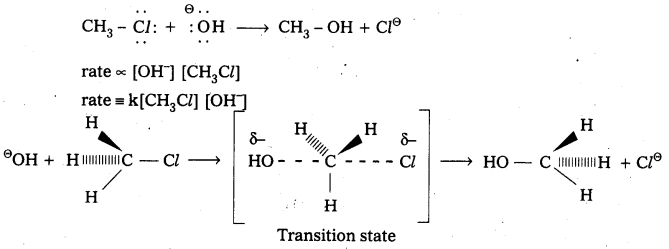
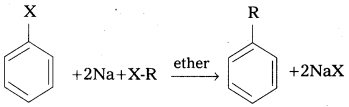
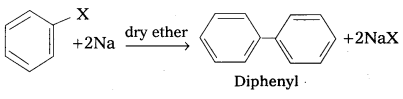
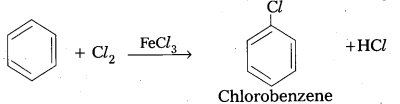
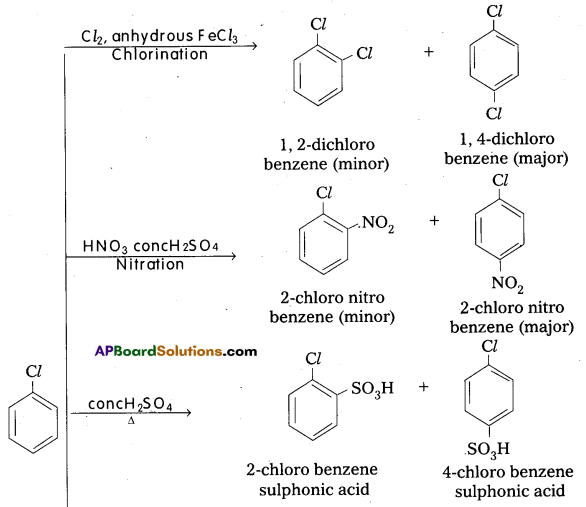
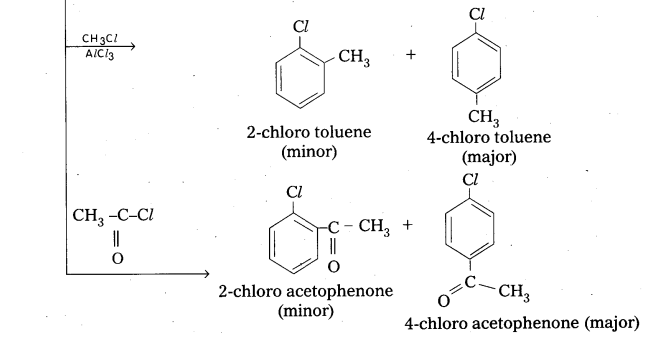
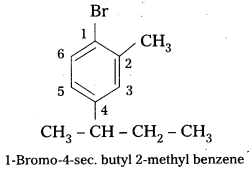
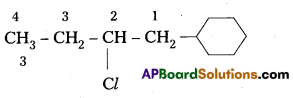
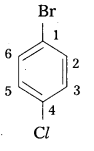
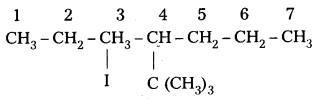

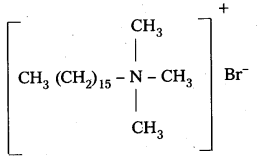
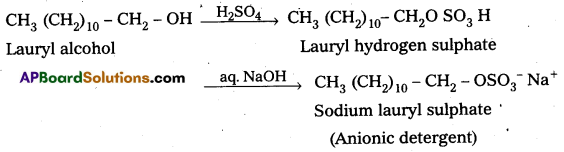
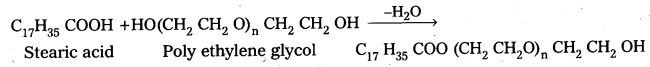
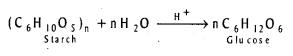
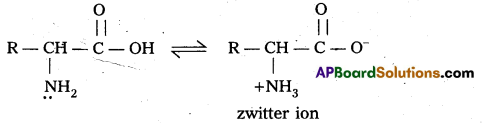
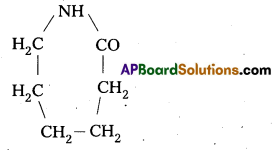
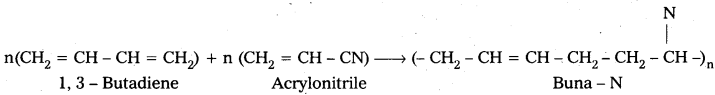
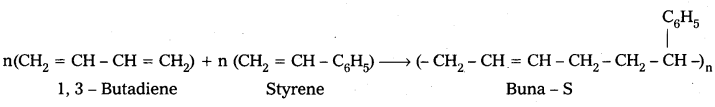
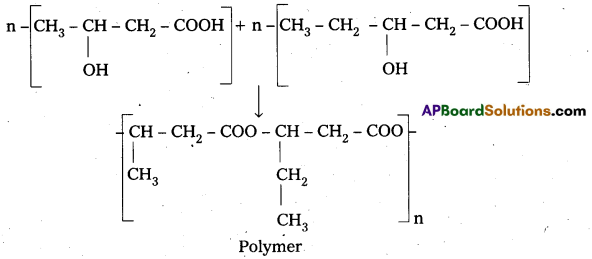
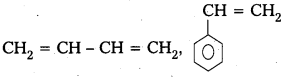
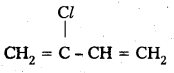
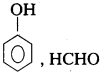
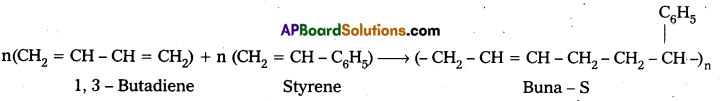
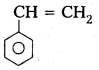 q
q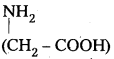 and amino caproic acid (H2N – (CH2)5 – COOH). It is a biodegradable polymer.
and amino caproic acid (H2N – (CH2)5 – COOH). It is a biodegradable polymer.