Students get through AP Inter 2nd Year Chemistry Important Questions 4th Lesson Surface Chemistry which are most likely to be asked in the exam.
AP Inter 2nd Year Chemistry Important Questions 4th Lesson Surface Chemistry
Very Short Answer Questions
Question 1.
What is adsorption? Give one example.
Answer:
Adsorption: The accumulation (or) concentration of a substance on the surface rather than in the bulk of solid (or) liquid is known as adsorption.
Eg : Adsorption of gases like O2, H2, Cl2 etc., on charcoal.
Question 2.
What is absorption ? Give one example.
Answer:
Absorption : The uniform distribution of a substance through out the bulk of the solid substance is known as absorption.
Eg : Chalk stick dipped in ink.
![]()
Question 3.
What is desorption ?
Answer:
Desorption : The process of removing an adsorbed substance from a surface on which it is adsorbed is called desorption.
Question 4.
What is sorption ? (or) What is the name given to the phenomenon when both absorption and adsorption take place together ?
Answer:
The process in which both adsorption and absorption takes place simultaneously is called sorption.
Question 5.
Give any two applications of adsorption.
Answer:
Applications of adsorption :
a) Separation of inert gases : Different noble gases adsorb at different temperatures on coconut charcoal. By this principle (adsorptipn) mixture of noble gas is separated by adsorption on coconut charcoal.
b) Gas masks : Gas mask is a device which consists of activated charcoal (or) mixture of adsorbents is used by coal miners to adsorb poisonous gases during breathing.
Question 6.
What is an adsorption isotherm ? Write the equation of Freundlich adsorption isotherm.
Answer:
Adsorption Isotherm : The variation in the amount of gas adsorbed by the adsorbent with pressure at constant temperature can be expressed by means of a curve known as adsorption isotherm.
- Freundlich adsorption isotherm equation is \(\frac{\mathrm{x}}{\mathrm{m}}\) = k. P1/n
x = mass of the gas adsorbed
m = mass of the adsorbent
P, k and n are constants.
![]()
Question 7.
Define “promoters” and “poisons” in the phenomenon of catalysis. ‘
Answer:
Promoters: The substances which enhance the activity of catalyst are known as promoters.
Poisons : The substances which decrease the activity of a catalyst are known as poisons.
Question 8.
What is homogeneous catalysis ? How is it different from heterogeneous catalysis ?
Answer:
Homogeneous Catalysis : The catalysis in which reactants and catalyst are in same phase is called Homogeneous catalysis.
- In case of heterogeneous catalysis, catalyst and reactants are present in different phases where as in case of homogeneous catalysis catalyst and reactants are present in same phase.
Question 9.
What are enzymes ? What is their role in human body ?
Answer:
Enzymes are complex nitrogenous organic compounds which are produced by living plants and animals.
- These act as specific catalysts in biological reactions.
- These catalyse the numerous reactions that occur in the bodies of animals and plants to maintain the life process.
Question 10.
Name any two enzyme catalyzed reactions. Give the reactions.
Answer:
1) Inversion of Cane Sugar :
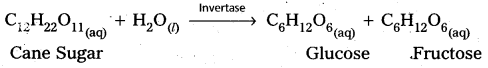
2) Decomposition of urea into ammonia and CO2 :
![]()
![]()
Question 11.
What is critical micelle concentration (CMC) and kraft temperature (Tk) ?
Answer:
The formation of micelles takes place only above a particular temperature called Kraft temperature (Tk) and above a particular concentration called Critical micelle concentration (CMC).
Question 12.
What is Peptization ?
Answer:
Peptization : The process of converting a precipitate into colloidal sol by shaking it with the dispersion medium in the presence of a small amount of electrolyte is called Peptization.
Question 13.
What is Brownian movement.
Answer:
Brownian movement: This is a kinetic property of colloidal solution.
When a colloidal solution is examined by ultramicroscope, the colloidal particles are seemed to be moving in a rapid zig-zag motion.
This rapid motion of colloidal particles is called Brownian movement.
This motion is due to unequal bombardment of colloidal particles by molecules of dispersion medium. Smaller the colloidal particles the more rapid is the Brownian movement.
Question 14.
What is Tyndall effect ?
Answer:
Tyndall effect : When light enters a colloidal solution, it is scattered by the large sized colloidal dispersed phase particles. Therefore when light passes through a solution we will be able to see the path of the light as a luminous beam. This is called Tyndall effect.
This is an optical property exhibited by colloidal solution. This phenomenon is clearly seen with a microscope placed at right angles to the path of light. Colloidal particles become self luminous due to absorption of light. A true solution does not show Tyndall effect.
Question 15.
What is electrokinetic potential or zeta potential ?
Answer:
In a colloidal sol the charges of opposite signs on the fixed and diffused parts of the double layer results in a difference in potential between these layers. The potential difference between the fixed layer and the diffused layer of opposite charge is called electro kinetic potential (or) zeta potential.
Question 16.
What is electrophoresis ?
Answer:
When electric potential is applied across two platinum electrodes dipping in a colloidal solution, the colloidal particles move towards one or the other electrode. The movement of colloidal particles under an applied emf is called “electrophoresis”.
![]()
Question 17.
What is coagulation ?
Answer:
The stability of the lyophilic sols is due to the presence of charge on the colloidal particles. If this charge is neutralised the particles will come nearer to each other to form aggregates (or coagulate) and settle down under the force of gravity. This process of settling downward colloidal particles is called coagulation (or) flocculation (or) precipitation.
Question 18.
State Hardy – Schulze rule.
Answer:
Greater the valence of the coagulating ion added, the greater is its power to cause coagulation. This is known as Hardy-Schulze rule.
Question 19.
What is protective colloid ?
Answer:
Lyophilic colloids used for the prevention of coagulation of lyophobic colloids are called protective colloids.
- Lyophilic colloids protect the lyophobic colloids. .
Question 20.
What is an emulsion ? Give two examples. (AP Mar. 17)
Answer:
Emulsion : The colloidal system in which a dispersion of finely divided droplets of a liquid in another liquid medium is called emulsion. Eg : Milk, Vanishing cream, Cold cream.
![]()
Question 21.
What is an emulsifying agent ?
Answer:
Emulsifying agent: The third substance which is added in small amounts to an emulsion to stabilize the emulsion is called emulsifying agent.
Question 22.
Name any two applications of colloidal solutions.
Answer:
Applications of colloidal solutions :
Rubber: Plant latex is a colloidal solution of rubber particles which are negatively charged. Rubber is obtained from latex by coagulation.
Industrial Products: Paints, inks, synthetic plastics, rubber, graphite, lubricants, cement, etc., are all colloidal in nature.
Question 23.
Define the terms adsorbate and adsorbent.
Answer:
The substance which is adsorbed is called adsorbate. The substance on whose surface the adsorption takes place is called adsorbent.
Question 24.
What is Gold number ?
Answer:
The number of milligrams of a protective colloid required to prevent the coagulation of 10ml Gold sol when 1 ml of 10% NaCl solution is added is called Gold number.
Short Answer Questions
Question 1.
What are different types of adsorption ? Give any four differences between characteristics of these different types. (IPE Mar & May 2015 (AP), (TS), 2016 (TS))
Answer:
Adsorption process is divided into two types.
- Physisorption
- Chemisorption.
Distinguishing characteristics of Physisorption and Chemisorption are given in the following table:
Physisorption
- This process is weak, due to Vander Waals forces.
- The process is reversible.
- This is a quick process i.e., takes place quickly.
- The process decreases with increase of temperature.
- This is a multilayered process.
- The process depends mainly on the nature of the adsorbent.
Chemisorption
- This process is strong, due to chemical forces.
- The process is irreversible.
- This is a slow process.
- The process increases with increase of temperature.
- This is a unilayered process.
- The process depends both on the nature of adsorbent and adsorbate.
![]()
Question 2.
What is catalysis ? How is catalysis classified? Give two examples for each type of catalysis. (IPE 2015 (AP), 14, BMP. 2016 (AP))
Answer:
Catalysis : A substance which alters the rate of a chemical reaction without itšelf being consumed in the process, is called a catalyst.
The action of catalyst in altering the rate of a chemical reaction is called catalysis
Types of catalysis : Catalysis is classified into two týpes as
a) Homogeneous catalysis and
b) Heterogeneous catalysis.
Homogeneous catalysis: The catalytic process in which the catalyst is present in the same phase as that of reactants, is known as homogeneous catalysis.
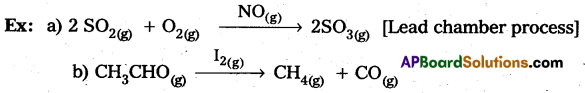
Heterogeneous catalysis : The catalytic process in which the catalyst is present in a phase different from that of the reàctants is known as heterogeneous catalysis.
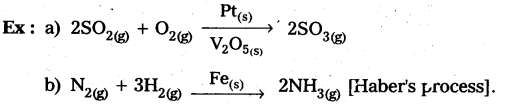
Question 3.
How can the constants k and n of the Freundlich adsorption equation be calculatéd?
Answer:
Freundlich adsorption isotherm equation is
\(\frac{x}{m}\) = k. P1/n ⇒ x/m = Extent of adsorption ⇒ P = Pressure
k and n are constants which depend on the nature of the adsorbent and the gas at a particular temperature.
Applying logarithm to the above equation
log \(\frac{x}{m}\) = log k + \(\frac{1}{n}\) log P.
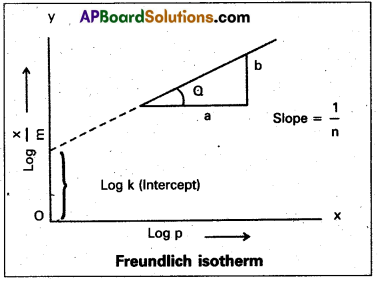
- A graph is plotted taking log \(\frac{x}{m}\) on y – axis and log P on x – axis. If the graph is a straight line then Freundlich isotherm is valid.
- The slope of the straight line gives \(\frac{1}{n}\) value.
- The intercept on the y-axis gives value of log k.
- \(\frac{1}{n}\) has values between 0 and 1.
When \(\frac{1}{n}\) = 0, \(\frac{x}{m}\) = constant, the adsorption is independent of pressure.
\(\frac{1}{n}\) = 1, \(\frac{x}{m}\) = kP i.e., \(\frac{x}{m}\) ∝ p.
Question 4.
How are colloids classified on thé basis of interaction between dispersed phase and dispersion medium?
Answer:
Lyophilic colloid : The colloidal solution in which the dispersed phase has great affinity to the dispersion medium is called a Lyophilic colloid or Lyophilic solution.
Ex: Starch solution.
The starch paste when dissolved in hot water, with stirring, the starch solution is formed. The starch particles (dispersed phase) has great affinity to water molecules (dispersion medium). So starch solution is a lyophilic solution or lyophilic colloid.
Lyophobic colloid : The colloidal solution in which there exists not much affinity between the dispersed phase and dispersion medium, it is called a Lyophobic colloid or Lyophobic solution.
Ex: Gold solution.
Gold rods are placed in water containing alkali. Electric arc is applied between gold rods. The gold particles dissolves in water, to give gold solution.
Gold particles (dispersed phase) have not much affinity towards water (dispersion medium). So this is a Lyophobic solution or Lyophobic colloid.
![]()
Question 5.
Explain any 2 methods for the preparation of colloids.
Answer:
Method – 1: Bredig’s arc Method : This process consists of dispersion and condensation colloids of Gold, Platinum, Silver arc prepared by this method. In this method an electric arc is struck between the electrodes of the metal immersed in the dispersion medium. The heat produced vapourises the metal which then condensed to colloidal size.
Method – 2 : Colloids can be prepared by chemical reactions like double decomposition, oxidation, reduction and hydrolysis.
Double decomposition : As2O3 + 3H2S → AS2S3 (sol) + 3H2O
Oxidation : SO2 + 2H2S → 3S(sol) + 2H2O
Reduction : 2 AuCl3 + 3 HCHO + 3 H2O → 2 Au (sol) + 3HCOOH + 6HCl
Hydrolysis : FeCl3 + 3H2O → Fe (OH)3 (sol) + 3HCl
Question 6.
Discuss the use of colloids in
i) Purification of drinking water
ii) Tanning
iii) Medicines.
Answer:
i) Purification of drinking water: The water obtained from natural sources often contains suspended impurities. Alum is added to such water to coagulate the suspended impurities and make water fit for drinking purposes.
ii) Tanning : Animal skins are colloidal in nature. When a skin, which has positively charged particles, is soaked in tannin, which contains negatively charged colloidal particles, mutual coagulation takes place. This results in the hardening of skin (leather). This process is termed as tanning. Chromium salts are also used in place of tannin.
iii) Medicines : Most of the medicines are colloidal in nature. For example argyrol is a silver sol used as an eye lotion. Colloidal antimony is used in curing kalaazar. Colloidal gold is used as intramuscular injection. Milk of magnesia an emulsion, is used for stomach disorders. Colloidal medicines are more effective because they have large surface area and are therefore easily assimilated.
Question 7.
What do you mean by activity and selectivity of catalysts?
Answer:
Activity:
The ability of a catalyst in increasing the rate of reaction is defined as activity of catalyst.
- The activity of a catalyst depends upon the strength of chemisorption to a large extent.
- The reactants must get adsorbed reasonably strongly onto the catalyst to become reactive.
Eg: The catalystic activity increases from Group – 5 to Group – 11 for hydrogenation reactions.
The maximum activity being shown by 7 – 9 group metals.
Selectivity:
The selectivity of a catalyst is its ability to direct a reacti6n to form specific products. The following reactions indicate the selectivity of heterogeneous catalysis.
- Starting with H2 and CO, and using different catalysts, we get different products,

The action of a catalyst is highly sélective in nature. A substance which acts as a catalyst in one reaction may fail to catalyse another reaction.
![]()
Question 8.
What are emulsions? How are they classified? Describe the applications of emulsion. ( AP Mar. 2017; IPE 2016 (TS))
Answer:
Emulsion : The colloidal system in which a dispersion of finely divided droplets of a liquid in another liquid medium is called emulsion.
Ex: Milk.
In Milk, the droplets of liquid fat are dispersed in water. This is an example for oil in water type emulsion.
Classification of emulsions: Emulsions are classified into two classes. These are
a) Oil in Water (O/W) and
b) Water in Oil (W/O), (O = Oil; W = Water).
These emulsions are classified as such depending on which is dispersed phase and which is dispersion medium.
a) Oil in Water (O/W) type emulsions : In this type of emulsions, the dispersed phase is oil and the dispersion medium is water.
Ex: Milk, liquid, fat (oil) in water.
Vanishing cream; fat in water.
b) Water In Oil (W/O) type emulsions : In this type of emulsions, the dispersed phase is water and the dispersion medium is oil.
Ex : Stiff greases : water in lubrication oils
Cod liver oil : water in cod liver oil
Applications of Emulsions : Emulsions are useful
- In the digestion of fats in intestines.
- In washing processes of clothes and crockery.
- In the preparation of lotions, creams, ointments in pharmaceutical and cosmetics.
- In the extraction of metals (froth floatation).
- In the conversion of cream into butter by churning.
- To break oil and water emulsions in oil wells.
- In the preparation of oily type of drugs for easy adsorption to the body.
Question 9.
What is adsorption? Explain different types of adsorptions with suitable examples.
Answer:
The process of concentration of molecules of a gas (or) liquid on the surface of another substance is called adsorption. Adsorption is of two types,
(a) Physical adsorption
(b) Chemical adsorption.
a) Physical adsorption: It is also called Vander waals adsorption. A very weak Vander forces of attraction exists between adsorbate and adsorbent. It is multi layered and non-selective.
Ex: Adsorption of inert gases on activated coconut charcoal.
b) Chemical adsorption: It is also called chemisorption. A very strong chemical forces of attraction exists between adsorbate and adsorbent. It is mono-layered and highly selective.
Ex: Adsorption of H2 gas on nickel surface.