Students get through AP Inter 2nd Year Chemistry Important Questions Lesson 6(c) Group-17 Elements which are most likely to be asked in the exam.
AP Inter 2nd Year Chemistry Important Questions Lesson 6(c) Group-17 Elements
Very Short Answer Questions
Question 1.
Interhalogen compounds are more reactive than the constituent halogens except for fluorine – Explain.
Answer:
Inter halogen compounds are more reactive than halogens. This is because X – X’ bond in interhalogens is weaker than X — X bond in halogens except F – F bond.
Question 2.
What is the use of ClF3?
Answer:
ClF3 is very useful fluorinating agent and it is used for the production of VF6 in the enrichment of U235.
U(s) + 3ClF3(l) → UF6(g) + 3ClF(g)
![]()
Question 3.
Why are halogens coloured?
Anšwer:
Halogens are coloured due to the absorption of radiations in visible region which results in the excitation of outer electrons to higher energy level. Halogens absorb different quanta of radiation and display different colours.
Question 4.
Write the reactions of F2 and Cl2 with water. (TS Mar. 17) (IPE Mar. ‘14)
Answer:
Fluorine produces O2 and O3 on passing through water.
3F2 + 3H2O → 6HF + O3
2F2 + 2H2O → 4HF + O2
Question 5.
Electron gain enthalpy of fluorine is less than that of chlorine – explain.
Answer:
The negative electron gain enthalpy of the fluorine is less than that of chlorine. This is due to small size of fluorine atom which results in strong inter electronic repulsions in the relatively small 2p orbitals of fluorine and thus the incoming electron does not experience much attraction.
Question 6.
Bond dissociation enthalpy of F2 is less than that of Cl2 – Explain.
Answer:
Bond dissociation enthalpy of F2 is less than that of Cl2
Explanation:
In F2 molecule electron repulsions are greater among lone pairs because these lone pairs are much closer to each other than in case of Cl2.
Question 7.
Write the formulae of the compounds, in which oxygen has positive oxidation states and mention the oxidation states of oxygen in them.
Answer:
In OF2 and O2F2 oxygen has positive oxidation states.
- In OF2, oxygen oxidation state is + 2.
- In O2F2 oxygen oxidation state is + 1.
![]()
Question 8.
What is the use of O2F2 and I2O5?
Answer:
Uses of O2F2
- O2F2 is a fluorinating agent. O2F2 oxidises plutonium to PUF6 and the reaction is used in removing plutonium as PUF6 from spent nuclear fuel.
Use of I2O5:
- I2O5 is a good oxidising agent and is used in the estimation of carbon monoxide (CO).
Question 9.
Explain the reactions of Cl2 with NaOH. (IPE – 2015 (AP), 2016 (TS), (AP))
Answer:
i) Reaction with cold dilute NaOH: Chlorine reacts with cold dilute NaOH to give sodium hypochlorite and sodium chloride.
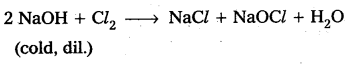
ii) Reaction with hot concentrated NaOH : Chlorine reacts with hot concentrated NaOH to give sodium chlorate and sodium chloride.

Question 10.
What happens when Cl2 reacts with dry slaked lime ? (AP Mar. 17: IPE 16, 15 (AP))
Answer:
Chlorine reacts with dry slaked lime and forms bleaching powder.
Ca(OH)2 + Cl2 → CaOCl2 + H2O.
Question 11.
What is aqua regia ? Write its reaction with gold and platinum.
Answer:
A mixture of 3 parts of conc. HCl and one part of Conc. HNO3 constitutes aqùa regia. It is used for dissolving noble metals.
It’s reaction with gold:
Au + 4H+ + \(\mathrm{NO}_3^{-}\) + 4Cl– → \(\mathrm{AuCl}_4^{-}\) + NO + 2 H2O
It’s reaction with Platinum:
3Pt + 16H+ + \(4 \mathrm{NO}_3^{-}\) + 18Cl– → 3PtC\(l_6^{-2}\) + 4NO + 8 H2O
![]()
Question 12.
How is chlorine manufactured by Deacon’s method? (AP Mar.’ 17: IPE ’16 (TS))
Answer:
Deacon’s process: [n Deacon’s process. Chlorine is obtained by the oxidation of hydrogen chloride gas by atmospheric oxygen in the presence of CuCl2 (catalyst) at 723 K.
![]()
Question 13.
Why is dry chlorine cannot act as a bleaching agent.
Answer:
Dry chlorine cannot produce nascent oxygen. Hence it cannot act as a bleaching agent.
Question 14.
HF is a liquid while HCl is a gas — explain.
Answer:
HF is a liquid due to the presence of inter molecular hydrogen bonding where as HCl is a gas as there is no such type of bonding in it.
Question 15.
Write two uses of hydrogen chloride.
Answer:
Uses of hydrogen chloride:
- It is used in medicines and as a laboratory reagent.
- It is used in the manufacturing of Cl2, NH4Cl and glucose (from corn starch).
- It is used in extracting glue from bones and purifying bone black.
Question 16.
Chlorine acts as an oxidizing agent – explain with two examples.
Answer:
Chlorine acts as oxidising agent.
Example – 1: Cl2 oxidises Iodine to Iodate.
I2 + 6 H2O + 5 Cl2 → 2HIO3 + 10 HCl
Example – 2: Cl2 oxidises Sodium Sulphite to Sodium Sulphate.
Cl2 + Na2SO3 + H2O → Na2SO4 + 2 HCl
Question 17.
Write the reaction of chlorine with hypo (Na2S2O3). (IPE Mar.2015 (AP, TS), 2016 (TS), (AP))
Answer:
Reaction of chlorine with hypo (Na2S2O3). Na2S2O3 + Cl2 + H2O → Na2SO4 + 2HCl + S. In this reaction hypo acts an “antichlor”.
![]()
Question 18.
Give the bond dissociation order of halogens.
Answer:
Bond dissociation order of halogens is, Cl2 > Br2 > F2 > I2.
Question 19.
I2 is more soluble in KI give reason.
Answer:
I2 forms soluble KI3 with KI solution. I2 + KI → KI3.
Question 20.
Chlorine acts as a bleaching agent only in the presence of moisture — explain.
Answer:
Moist chlorine is a powerful bleaching agent. This bleaching property is due to oxidation.
Cl2 + H2O → 2HCl + (O)
Ex: Coloured substance + (0) → colourless substance.
Question 21.
The decreasing order of acidic character among hypohalogen acids is HClO > HBrO > HIO. Give reason.
Answer:
Given the decreasing order of acidic character among hypohalogen acids is
HClO > HBrO > HIO
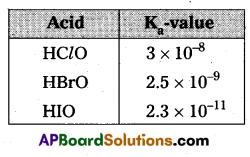
From the above mentioned Ka values the given order of hypohalogen acids – acid strength order is
HClO > HBrO > HIO
Question 22.
fluorine exhibits only-1 oxidation state whereas other halogens exhibit +1, +3, +5 and +7 oxidation states also. Explain.
Solution:
Fluorine is the most electronegative element and cannot exhibit any positive oxidation state. Other halogens have d- orbitais and therefore, can expand their octets and show +1, +3, +5 and +7 oxidation states also.
![]()
Question 23.
What are the inter halogen compounds ? Give two examples.
Answer:
The compounds formed between different halogens are called inter halogen compounds.
Ex: ClF3, BrF3, IF7, ICl3.
Short Answer Questions
Question 1.
Explain the structures of
a) BrF5 and
b) IF7.
Answer:
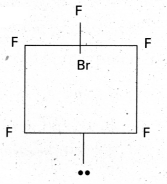
a) Structure of BrF5.
- Central atom in BrF5 is ‘Br’
- ‘Br’ undergoes sp3d2 hybridisation in 2nd excited state.
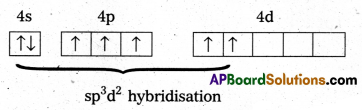
- Shape of the molecule is octahedral with one position occupied by a lone pair (or) square pyramidal.
b) Structure of IF7:
- Central atom in IF7 is 1’.
- ‘I’ undergoes sp3d3 hybridisation iñ 3rd excited state.
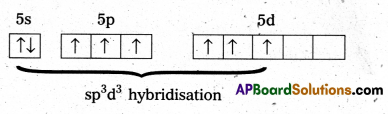
- Shape of the molecule is Pentagonal bipyramid structure.
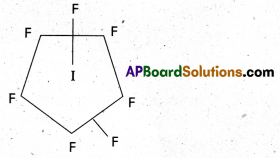
Question 2.
How is chlorine obtained in the laboratory? How does it react with the following?
a) cold dil. NaOH
b) excess NH3
c) KI (IPE Mar & May – 2015 AP TS), BMP)
Answer:
In the laboratory chlorine is prepared by the oxidation of HCl with MnO2.
4 HCl + MnO2 → MnCl + 2 H2O + Cl2↑
a) Chlorine reacts with cold dil. NaOH to form sodium hypochlorite.
Cl2 + 2 NaOH → NaCl + NaOCl + H2O
b) Cl2 reacts with excess of NH3, Nitrogen and ammonium chloride are formed.
8 NH3 + 3 Cl2 → 6 NH4Cl + N2↑
c) Cl2 reacts with KI to liberate iodine.
Cl2 + 2KI → 2KCl + I2↑
![]()
Question 3.
How is ClF3 prepared? How does it react with water? Explain its structure.
Answer:
Preparation of ClF3: Chlorine reacts with excess of fluorine to form ClF3.
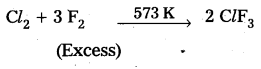
Reaction with H2O : ClF3 reacts with water explosively and oxidises water to give oxygen or in controlled quantitieš oxygen diflouride (OF2) as well as HF and HCl.
ClF3 + 2 H2O → 3 HF + HCl + O2
ClF3 + H2O → HF + HCl + OF2
Structure of ClF3:
- Central atom in ClF3 is ‘Cl’
- Excited state electronic configuration of ‘Cl’ is
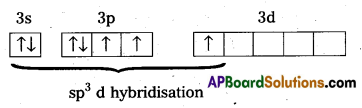
- Cl’ atom undergoes sp3d hybridisation.
- It is a bent T-shaped molecule (or) trigonal bipyramidal with 2 – positions occupied by lone pairs.
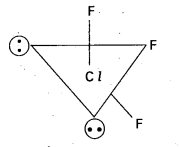
Question 4.
How can you prepare Cl2 from HCl and HCl from Cl2 ? Write the reactions.
Answer:
Preparation of Cl2 from HCl:
- On heating MnO2 with conc. HCl, Cl2 gas is liberated.
MnO2 + 4 HCl → MnCl2 + Cl2 + 2 H2O - By the oxidation of HCl gas by atmospheric oxygen in presence of CuCl2 catalyst at 723K.
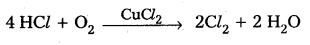
Preparation of HCl from Cl2: - Cl2 when reacted with H2 to form HCl,
H2(g) + Cl2(g) → 2HCl(g)
Question 5.
How is chlorine prepared by electrolytic method? Explain Its reaction with
a) NaOH and
b) NH3 under different conditions. (IPE Mar & May – 2015 (AP, TS))
Answer:
Preparation of chlorine by electrolytic method: Chlorine is obtained by the electrolysis of brine solutions (Cone. NaCl). Cl2 gas is liberated at anode.
2 NaCl → 2 Na+ + 2Cl–
2 H2O + 2e– → 2 OH– + H2 (cathode)
2 Cl– → Cl2 + 2e– (anode) .
a)
i)NaOH:
Chlorine reacts with cold dil. NaOH to form sodium hypochlorite.
Cl2 + 2 NaOH → NaCl + NaOCl + H2O
ii) Cl2 reacts with hot conc. NaOH to form sodium chloride and sodium chlorate.
3 Cl2 + 6 NaOH → 5 NaCl + NaClO3 + 3 H2O
b)
i) Cl2 reacts with excess of NH3, Nitrogen and ammonium chlorate are formed.
8 NH3 + 3 Cl2 → 6 NH4Cl + N2↑
ii) NH3 reacts with excess of Cl2 to form NCl3 and HCl.
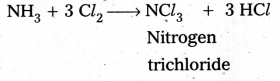
Question 6.
What are interhalogen compounds? Give some examples to illustrate the definition. How are they classified?
Answer:
The binary diamagnetic compounds of halogens which are formed by the reaction of halogens among themselves are called interhalogen compounds.
E.g.: IF7, ClF3, BrF3, ClF, IF3 etc.
The above examples are binary diamagnetic compounds and formed by combination of halogens only.
Inter halogen compounds are classified into four types.
- AX – Týpe :Eg: ClF, BrF
- AX3 – Tÿpe : Eg : ClF3, IF3
- AX5 – Type : Eg: ClF5, BrF5
- AX7 – Type : Eg : IF7
- ‘A’ is less electronegative halogen.
- X is more electronegative halogens.
Question 7.
Write the names and formulae of the oxoacids of chlorine. Explain their structures and relative acidic nature.
Answer:
Four oxyacids of chlorine are known. They are
Hypochlorous acid – HOCl
Chlorous acid — HClO2
Chloric acid – HClO3
Perchloric acid – HClO4
Structure of HClO: In this chlorine atom is sp3 hybridised. Outer electronic configuration of Cl in ClO– after sp3 hybridisation.
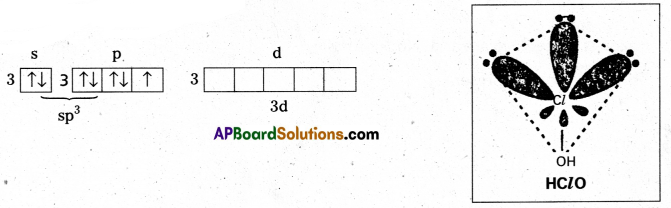
Shape is tetrahedral with 3 lone pairs (or) linear
No π — bonds.
Chlorous acid: (HClO2) : Chlorine is in sp3 hybrid state, in first excited state. Shape is tetrahedral with 2 lone pairs (or) angular one πd-p bond is present.
First excited state
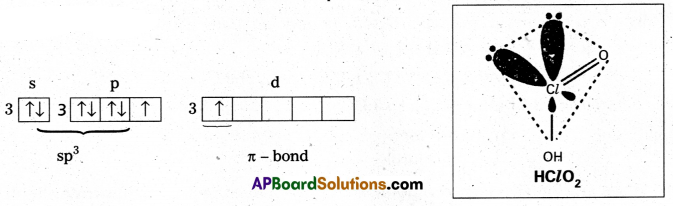
Chloric acid (HClO3) : The central chlorine atom undergoes sp3 hybridisation in second excited state.
Second excited state
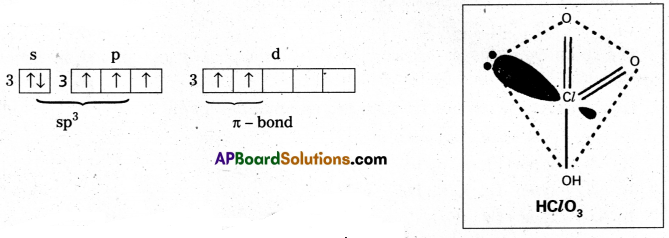
Shape is tetrahedral with one lone pair (or) pyramidal.
Two πd-p bonds present.
Perchloric acid (HClO4) : The central chlorine atom undergoes sp3 hybridisation in third excited state.
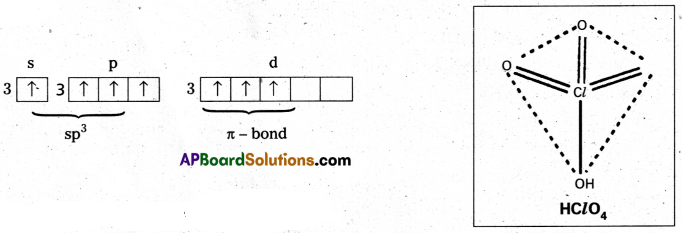
Shape is perfect tetrahedral. No lone pairs.
Three πd-p bonds present.