Students get through AP Inter 2nd Year Chemistry Important Questions 8th Lesson Polymers which are most likely to be asked in the exam.
AP Inter 2nd Year Chemistry Important Questions 8th Lesson Polymers
Very Short Answer Questions
Question 1.
What are polymers ? Give example.
Answer:
Polymer : A large molecular weight complex compound which is formed by the repeated combination of simple units (monomers) is called polymer. E.g.: Nylon 6, 6, Buna-S,. rubber etc.
Question 2.
What is polymerization ? Give an example of polymerization reaction. [IPE 2014 Mar. 14]
Answer:
Polymerization: The process of formation of polymers from respective monomers is called polymerization.
(or)
A large molecular weight complex compound which is formed by the repeated combination of simple units is called polymer. This process is called polymerisation.
E.g. : Formation of polyethene from ethene and reaction of hexamethylene diamine and adipic acid leading to the formation of Nylon 6, 6 are examples of two different types of polymerisation reactions.
![]()
Question 3.
What is addition polymer ? Give example. [IPE Mar. 2015 (TS)]
Answer:
Addition Polymer: The polymer which is formed by the addition of molecules of monomers of same type (or) different type containing double bonds is called addition polymer.
E.g. : Polyethene, poly acrylonitrile.
Question 4.
What is condensation polymer ? Give example.
Answer:
Condensation polymer : The polymer which is formed by the condensation reaction between molecules having more than one functional group is called condensation polymer.
E.g. : Nylon 6, 6, Poly ethylene terephthalate.
Question 5.
What are homopolymers ? Give example.
Answer:
Homopolymers : The polymers which are formed by the polymerisation of a single monomeric species are known as homopolymers.
E.g.: Polyethene, Poly styrene.
Question 6.
What are copolymers ? Give example. [IPE 2014]
Answer:
Copolymers: A polymer which is formed by the polymerisation of two (or) more chemically different types of monomer units is called copolymer.
E.g.: Butadiene – Styrene polymer (Buna-S)
Question 7.
What are elastomers. Give example.
Answer:
Elastomers : There are rubber like solids with elastic properties. In elastomers the polymer chains are held together by the weak enter molecular forces.
E.g. : Buna – S, Buna – N etc.
![]()
Question 8.
What are fibres ? Give example.
Answer:
Fibres : Fibres are the thread forming solids which possess high tensile strength.
E.g. : Nylon 6, 6, polyesters.
Question 9.
What are thermoplastic polymers ? Give example.
Answer:
Thermoplastic Polymers: These are the linear (or) slightly branched long chain molecules capable of softening on heating and hardening on cooling.
E.g. : Polystyrene, polythene.
Question 10.
What are thermosetting polymers ? Give example.
Answer:
Thermo setting polymers: These polymers are cross linked (or) heavily branched molecules which on heating undergo extensive cross linking in moulds and again become infusible.
E.g. : Bakelite, urea-formaldehyde resin etc.
Question 11.
What is Ziegler – Natta catalyst ?
Answer:
A mixture of Tri alkyl aluminium and titanium chloride is called Ziegler – Natta catalyst
E.g. : (C2H5)3 Al + TiCl4
Question 12.
What are the repeating monomeric units of Nylon 6 and Nylon 6, 6 ?
Answer:
The repeating monomeric units of Nylon – 6 is Capro lactum.
The repeating monomeric units of Nylon 6, 6 are hexamethylene diamine and Adipic acid.
H2N-(CH2)6 – NH2
Hexamethylene
HOOC – (CH2)4 – COOH
Adipic acid
![]()
Question 13.
What is the difference between Buna – N and Buna – S ?
Answer:
Buna – N : Buna – N is the copolymer which is formed by the polymerisation of 1, 3 – Butadiene and acrylonitrile.
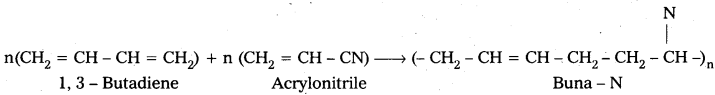
Buna – S : Buna – S is the copolymer which is formed by the polymerisation of 1, 3 – Butadiene and Styrene.
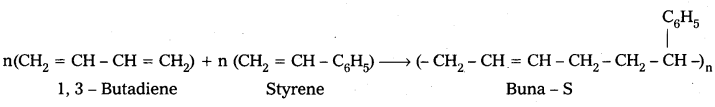
Question 14.
What is PDI (Poly Dispersity Index) ?
Answer:
Poly Dispersity Index (PDI) : The ratio between weight average molecular mass (\(\overline{\mathrm{M}}_{\mathrm{w}}\)) and the number average molecular mass (\(\overline{\mathrm{M}}_{\mathrm{n}}\)) of a polymer is called Poly Dispersity Index (PDI).
Question 15.
What is vulcanization of rubber ? [A.P. Mar. 17] [IPE – 2016 (TS)]
Answer:
Vulcanization of rubber : The process of heating the raw rubber with sulphur (or) with sulphur compounds to improve it’s physical properties is called vulcanization of rubber.
Question 16.
What is biodegradable polymer ? Give one example of a biodegradable polyester ?
Answer:
Biodegradable polymers : The polymers degradable by enzymatic hydrolysis and to some extent by oxidation are called biodegradable polymers.
Eg.: Nylon – 2, Nylon – 6, PHBV, Polyglycolic acid, Polylactic acid etc.
![]()
Question 17.
What is PHBV ? How is it useful to man ? [IPE Mar – 2015 (TS), B.I.E, 2016 (TS)]
Answer:
Poly β – hydroxy butyrate – CO – β – hydroxy Valerate (PHBV) : It is a Copolymer of 3 -hydroxy butanoic acid and 3 – hydroxy pentanoic acid.
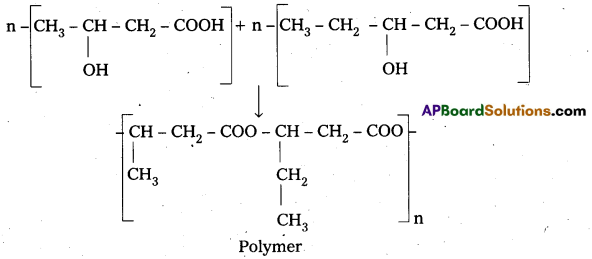
Properties & Uses : The properties of PHBV vary according to the ratio of both the acids, 3-hydroxy butanoic acid provides stiffness and 3-hydroxy pentanoic acid imparts flexibility to copolymer.
It is used in medicine for making capsules.
PHBV also undergoes degradation by bacteria.
Short Answer Questions
Question 1.
Write the names and structures of the monomers of the following polymers. [A.P. Mar. 17]
i) Buna – S
ii) Buna – N
iii) Dacron
iv) Neoprene
Ans:
i) Buna – S
Monomers : 1, 3 – Butadiene, Styrene
Structure :
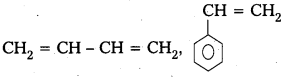
ii) Buna-N
Monomers : 1, 3 – Butadiene, Acrylonitrile
Structure : CH2 = CH – CH = CH2, CH2 = CH – CN
iii) Dacron
Monomers : Ethylene glycol, Terephthalic acid
Structure : HOCH2 – CH2OH, HOOC ![]() COOH
COOH
iv) Neoprene .
Monomers : 2 – chloro – 1, 3 – Butadiene
Structure :
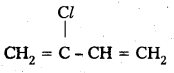
Question 2.
Define thermoplastics and thermosetting polymers with two examples of each.
Answer:
Thermoplastic Polymers: These are the linear (or) slightly branched long chain molecules capable of softening on heating and hardening on cooling. E.g.: Polystyrene, polythene.
Thermo setting polymers: These polymers are cross linked (or) heavily branched molecules which on heating undergo extensive cross linking in moulds and again become infusible.
Eg.: Bakelite, urea-formaldehyde, resin etc.
![]()
Question 3.
Distinguish between the terms homo polymer and co polymer. Give one example of each.
Answer:
Homopolymers : The polymers which are formed by the polymerisation of a single monomeric species are known as homopolymers.
E.g.: Polyethene, Poly styrene.
Copolymers: A polymer which is formed by the polymerisation of two (or) more chemically different types of monomer units is called copolymer.
E.g.: Butadiene – Styrene polymer (Buna-S)
Question 4.
Explain the purpose of vulcanization of rubber. [T.S. Mar. 17]
Answer:
- Natural rubber becomes soft at high temperatures and brittle at low temperatures. It shows high water absorption capacity.
- Natural rubber is soluble in non-polar solvents and is non-resistant to oxidising agents.
- To improve these physical properties rubber can be vulcanised.
- The process of heating the raw rubber with sulphur (or) with sulphur compounds to improve it’s physical properties is called vulcanisation of rubber.
- The vulcanized rubber has improved properties like elasticity, minimum water absorbing tendency, high resistance to chemical oxidation as well as organic solvents.
Question 5.
Write the names and structures of the monomers used for getting the following polymers [A.P. Mar. 17; Mar. 14]
i) Polyvinyl chloride
ii) Teflon
iii) Bakelite
iv) Polystyrene.
Answer:
i) Polyvinyl chloride .
Monomer: Vinyl chloride
Structure : CH2 = CH – Cl
ii) Teflon
Monomer: Tetrafluoro ethylene
Structure : CF2 = CF2
iii) Bakelite [IPE 2015 (AP), 2014, B.M.P, 2016 (AP)
Monomers : Phenol, Formaldehyde
Structure :
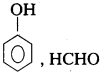
iv) Polystyrene
Monomer : Styrene
Structure :
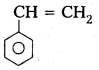 q
q
Question 6.
Name the different types of molecular masses of polymers.
Answer:
The different types of important molecular masses of polymers are
- Number average molecular mass (\(\overline{\mathbf{M}}_{\mathrm{n}}\))
- Weight average molecular mass (\(\overline{\mathbf{M}}_{\mathrm{w}}\))
Question 7.
What is natural rubber ? How does it exhibit elastic properties ?
Answer:
- Natural rubber is a polymer and possesses elastic properties.
- It is an elastomer and it is manufactured from rubber latex. Latex is a colloidal dispersion of rubber in water.
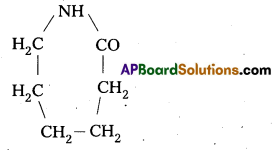
- Natural rubber may be considered as a linear polymer of isoprene. It is also called as cis-1, 4-Poly isoprene.
- The cis poly isoprene molecule consists of various chains held together by weak vander waals interactions and has a colloid structure. Thus it stretches like a spring and exhibits elastic properties.
![]()
Question 8.
Give the structure of nylon 2 – nylon 6 ?
Answer:
Nylon 2 – Nylon 6 :
It is an alternating polyamide copolymer of glycine 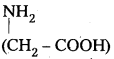 and amino caproic acid (H2N – (CH2)5 – COOH). It is a biodegradable polymer.
and amino caproic acid (H2N – (CH2)5 – COOH). It is a biodegradable polymer.
Structure of Nylon 2 – Nylon – 6
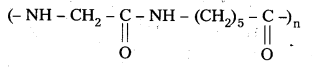
Question 9.
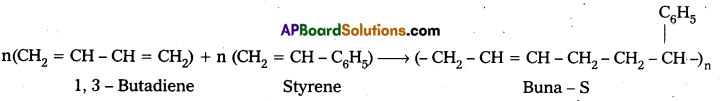
Explain copolymerization with an example. [IPE Mar – 2015 (AP), B.I.E.]
Answer:
Copolymers: A polymer which is formed by the polymerisation of two (or) more chemically different types of monomer units is called copolymer. E.g.: Butadiene-Styrene polymer(Buna-S) The process of formation of copolymer is called copolymerisation.
E.g. : Buna – S : Buna – S is the copolymer which is formed by the polymerisation of 1, 3 – Butadiene and Styrene.
Question 10.
What are LDP and HDP ? How are they formed ?
Answer:
Polythenes are two types :
- LDP (Low Density Polythene),
- HDP (High Density Polythene)
1) Low Density Polythene : LDP is formed by the polymerisation of ethene under high pressure of 1000 to 2000 atm. at a pressure of 350 to 570K in the presence of traces of dioxygen (or) a peroxide initiator.
Properties :
a) This is obtained through the free radical additon.
b) LDP is chemically inert and tough.
c) LDP is flexible and a poor conductor of electricity.
Uses:
a) It is used in the insulation of electric cables.
b) It is used in the manufacture of pipes in agriculture irrigation.
2) High Density Polythene : HDP is formed by the polymerisation of ethene in a hydro carbon solvent in presence of Ziegler Natta catalyst at a temperature of 333K to 343 K and under a pressure of 6 – 7 atm.
Properties :
a) HDP consists of linear molecules and has high density due to close packing.
b) It is chemically inert and more tough, hard.
Uses:
a) It is used in manufacture of house hold articles like buckets, dustbins etc.
b) It is used in manufacture of pipes.
![]()
Question 11.
What are natural and synthetic polymers ? Give two examples of each type.
Answer:
Natural polymers : The polymers which are obtained from natural sources such as plants and animals are called natural polymers.
E.g. : Starch, cellulose, rubber etc.
Synthetic polymers : The polymers which are artificially prepared i.e., man-made are called synthetic polymers.
These have wide applications in daily life as well as in industry.
E.g. : Plastics, Nylon 6, 6, synthetic rubbers.