Students get through AP Inter 2nd Year Chemistry Important Questions 7th Lesson d and f Block Elements & Coordination Compounds which are most likely to be asked in the exam.
AP Inter 2nd Year Chemistry Important Questions 7th Lesson d and f Block Elements & Coordination Compounds
Very Short Answer Questions
Question 1.
What are transition elements? Give examples.
Answer:
Transition elements are the elements that contain partially filled d-subshells in their ionic state (or) in their elementary state. Eg : Mn, Co, Ag etc.
Question 2.
Write the general electronic configuration of transition elements.
Answer:
General electronic configuration of transition elements is (n – 1)d1-10 ns1-2.
![]()
Question 3.
Why are Mn2+ compounds more stable than Fe2+ towards oxidation to their +3 state ?
Answer:
Mn+2 has electronic configuration [Ar] 4s0 3d5.
Fe+2 has electronic configuration [Ar] 4s0 3d6.
- Mn+2 has half filled d-subshell which is more stable.
Hence Mn+2 compounds are more stable than Fe+2 toward oxidation to their +3 state.
Question 4.
Why Zn2+ is diamagnetic whereas Mn2+ is paramagnetic ?
Answer:
- Zn+2 electronic configuration is [Ar] 4s03d10. It has no unpaired electrons. So it is dia magnetic.
- Mn+2 electronic configuration is [Ar] 4s03d5. It has five unpaired electrons so it is paramagnetic.
Question 5.
Write ‘spin only’ formula to calculate the magnetic moment of transition metal ions.
Answer:
Spin only formula to calculate the magnetic moment of transition metal ions is
μ = \(\sqrt{n(n+2)}\) BM BM = Bohr Magneton.
![]()
Question 6.
Claculate the ‘spin only’ magnetic moment of \(\mathrm{Fe}^{2+}(\mathrm{aq})\) ion. (Board Model Paper) (AP Mar. ’17)
Answer:
Fe+2 ion has electronic configuration [Ar] 4s03d6.
It has four unpaired electrons n = 4.
Spin only magnetic moment μ = \(\sqrt{n(n+2)}\) BM = \(\sqrt{4(4+2)}\) = \(\sqrt{24}\) BM = 4.89 BM
Question 7.
Aqueous Cu2+ ions are blue in colour, where as aqueous Zn2+ ions are colourless. Why ?
Answer:
- Electronic configuration of Cu+2 ion is [Ar] 4s03d9. It contains one unpaired electron due to presence of this unpaired electron aq. Cu+2 ions are blue in colour.
- Electronic configuration of Zn+2 ion is [Ar] 4s03d10. It contains no unpaired electrons, due to absence of unpaired electrons aq. Zn+2 ions are colourless.
Question 8.
What are complex compounds ? Give examples.
Answer:
Complex compounds : Transition metal atoms or ions form a large number of compounds in which anions or neutral groups are bound to metal atom or ion through co-ordinate covalent bonds. Such, compounds are called co-ordination compounds (or) complex compounds.
Eg. : [Fe(CN)6]4-, [Co(NH3)6]3+
Question 9.
What is an alloy? Give example.
Answer:
Alloy : An intimate mixture having physical properties similar to that of the metal formed by a metal with other metals or metalloids or sometimes a non metal is called as an alloy.
Eg.: Invar — 64% Fe, 35% Ni, Mn 8cc in traces
Nichrome — 60% Ni,, 25% Fe, 15% Cr.
Question 10.
What is lanthanoid contraction ? (IPE 2016(TS))
Answer:
The slow decrease of atomic and ionic radii in lanthanides with increase in atomic number is called lanthanide contraction.
![]()
Question 11.
What is mischmetall ? Give its composition and uses. (AP Mar. ’16)
Answer:
Mischmetall is an alloy which consists of a lanthanoid metal (~ 95%) and iron (~ 5%) and traces of S, C, Ca and Al.
- It is used in Mg- based alloy to produce bullets, shell and lighter flint.
Question 12.
What is a coordination polyhedron ?
Answer:
The spatial arrangement of the ligands which are directly bonded to the central atom or ions defines the geometry about the central atom is called co-ordination polyhedron.
Eg.: Octahedral, tetrahedral etc.
Question 13.
What is a ligand ? (TS Mar. ’18) (IPE 2014)
Answer:
Ligand : A co-ordinating entity which is bound to the central atom by donating electron pairs is called a ligand. Eg.: Cl–, NH3, CN– etc.
Question 14.
What is a chelate ligand ? Give example.
Answer:
The ligands which can form two co-ordinate covalent bonds through two donor atoms are called bidentate ligands. These bidentate ligands are also called chelate ligands.
Eg.: C2\(\mathrm{O}_4^{-2}\), \(\mathrm{CO}_3^{-2}\) etc.
Question 15.
What is an ambidentate ligand ? Give example. (TS Mar. ’18) (IPE 2016(AP))
Answer:
A unidentate ligand containing two possible donor atoms can co-ordinate through either of donor atoms. Such ligands are called ambidentate ligands. Eg : \(\mathrm{NO}_2^{-}\)
Question 16.
[Cr(NH3)6]3+ is paramagnetic while [Ni(CN)4]2- is diamagnetic. Why ?
Answer:
- [Cr(NH3)6]3+ is paramagnetic due to the presence of three unpaired electrons.
- [Ni(CN)4]2- is diamagnetic due to the absence of unpaired electrons.
![]()
Question 17.
Which metal in the first series of transition metals exhibits +1 oxidation state most frequently and why ?
Answer:
Copper exhibits +1 oxidation state most frequently because Cu+ has electronic configuration [Ar] 4s03d10 which has a fulfilled d-subshell which is more stable.
Question 18.
Why do transition elements exhibit more than one Oxidation state (variable oxidation states) ?
Answer:
Transition elements exhibits more than one oxidation state.
Reason :
- The energy difference between (n – 1) d subshell and ns subshell is very low. So both of these subshells complete to lose the electrons.
Question 19.
CuSO4.5H2O is blue in colour where as anhydrous CuSO4 is colourless. Why ? (IPE 2014)
Answer:
CuSO4.5H2O is blue in colour whereas anhydrous CuSO4 is colourless because in the absence of ligand, crystal field splitting doesnot occurs.
Question 20.
Give the formula the following complexes.
a) Potassium hexacyano ferrate (III)
b) Tetra carbonyl nickel (O) (IPE 2016 (TS))
Answer:
a) K3 [Fe (CN)6]
b) [Ni(CO)4]
Question 21.
Scandium is a transition element. But Zinc is not. Why ? (IPE 2014)
Answer:
Scandium has electronic configuration [Ar] 4s23d1.
Zinc has electronic configuration [Ar] 4s23d10.
Scandium has one unpaired d-electron where as Zinc has zero unpaired d-electrons so Scandium is transition element but Zinc is not.
Question 22.
What are interstitial compounds ? (IPE 2015)
Answer:
Interstitial compounds are non-stichiometric compounds which are formed when non-metals like H, C, N etc., are heated with transition elements. In these compounds the non-metal atoms occupy the interstitial species of metal atoms. Ex : TiH0.54
Question 23.
In what way is the electronic configuration of transition elements different from non transition elements ?
Answer:
- The general electronic configuration of transition elements is (n – 1) d1-10 ns1-2.
- The general electronic configuration of non-transition elements is (n – 1)d10 ns2.
Question 24.
Even though silver has d10 configuration, it is regarded as transition element. Why ?
Answer:
The outer electronic configuration of silver is – 4d105s1. It is having general electronic configuration of a transition element (n – 1) d1-10 ns1-2.
So silver is a transition element.
Question 25.
Though Sc is a transition element, it does not exhibit variable oxidation state. Why ?
Answer:
Scandium has electronic configuration [Ar] 4s23d1. It has only one unpaired d-electron. So it does not exhibits variable oxidation state. It exhibits +3 stable oxidation state.
![]()
Question 26.
Why is it difficult to obtain M3+ oxidation state in Ni, Cu and Zn ?
Answer:
- Ni has electronic configuration [Ar] 4s23d8.
Ni+2 has electronic configuration [Ar] 4s03d8.
It is difficult to remove an electron from 3d8. So, Ni+3 is difficult to obtain. (Ni has high negative enthalpy of hydration). - Cu has electronic configuration [Ar] 4s13d10.
Cu+ has electronic configuration [Ar] 3d10.
It is difficult to remove the electrons from 3d10 (stable). So, Cu+3 is difficult to obtain. - Zn has electronic configuration [Ar] 4s23d10.
Zn+2 has electronic configuration [Ar] 4s03d10.
It is difficult to remove the electron from 3d10 (stable). So Zn+3 is difficult to obtain.
Question 27.
Cu+2 forms halides like CuF2, CuCl2 and CuBr2 but not CuI2. Why ?
Answer:
Cu+2 forms halides like CuF2, CuCl2 and CuBr2 but not CuI2 because Cu+2 oxidises I– to I2.
2Cu+2 + 4I– → Cu2I2 + I2.
Question 28.
Why is Cr2+ reducing and Mn3+ oxidizing even though both have the same d4 electronic configuration ?
Answer:
Cr+2 is reducing as its configuration changes from d4 to d3, the latter having a half filled t2g level. On the other hand the change from Mn+2 to Mn+3 results in the half filled (d5) configuration which has extra stability.
Question 29.
What is meant by ‘disproportionation’ ? Give an example of disproportionation reaction in aqueous solution.
Answer:
The reactions in which only one element undergo both oxidation as well as reduction are called disproportionation reactions.
- When a particular oxidation state becomes less stable relative to other oxidation states, one lower, one higher, it is said to undergo disproportionation.
Eg: Cu+ ion is not stable in aqueous solution because it undergo disproportionation in aqueous solution.
![]()
Question 30.
Why do the transition metals readily form alloys?
Answer:
Because of similar atomic or ionic radii and similar characterstic properties of transition elements alloys are readily formed by these elements.
Question 31.
How do the Ionic character and acidic nature vary among the oxides of first transition series?
Answer:
- As the oxidation number of a metal increases ionic character decreases in case of transition elements. Eg: Mn2O7 is a covalent green oil.
- In CrO3 and V2O5 the acidic character is predominant.
- V2O5 is however amphoteric though mainlý acidic V2O5 reacts with alkali as well as acids to give \(\mathrm{VO}_4^{-3}\) and \(\mathrm{VO}_4^{-3}\).
- CrO is basic.
- Mn2O7 gives HMnO4 and CrO3 gives H2CrO4 and H2Cr2O7.
![]()
Question 32.
What are the different oxidation s.tates exhibited by the lanthanoids?
Answer:
- Lanthanoids exhibits +2, +3 states majorly. Sometimes +2 and +4 states exhibited in solid compounds.
- The common oxidation state of lanthanoids is +3.
Question 33.
What is difference between a double salt and a complex compound?
Answer:
Double salts dissociate into simple ions completely when dissolved in water while complex compounds dissociate to give complex ion and the counter ions.
Question 34.
What is meant by coordination number.
Answer:
Co-ordination Number is the number of ligands present around a central metal atom or ion in a complex Ex : The co-ordination number of Co in [Co(NH3)3 Cl3] is six.
Question 35.
Using IUPAC norms write the systematic names of the following.
- [CO(NH3)6]Cl3
- [Pt(NH3)2Cl(NH2CH3)]Cl
- [Ti(H2O)6]3+ and
- [NiCl4]2-
Answer:
- Hexa amine cobalt (III) chloride
- Diamine chloride (methyl amine) platinum (II) chloride
- Hexa aquo titanium (III) ion
- Tetra chloro nickelate (III) ion
Question 36.
Calculate the magnetic moment of a divalent ion in aqueous solution if its atomic number is 25. (IPE 2016 (AP)) (AP Mar. ’16)
Solution:
With atomic number 25, the divalent ion in aqueous solution will have d5 configuration (five unpaired electrons). The magnetic moment, μ is
μ = \(\sqrt{5(5+2)}\) = 5.92 BM
Short Answer Questions
Question 1.
Write the characteristic properties of transition elements.
Answer:
Transition elements exhibits typical characteristic properties.
- Electronic configurations.
- Para and ferro magnetic properties.
- Alloy forming ability.
- Complex forming ability.
- Interstitial compounds.
- Variable oxidation states.
- Formation of coloured hydrated ions.
- Catalytic property.
- Metallic character.
![]()
Question 2.
What is lanthanoid contraction ? What are the consequences of lanthanoid contraction ? (AP Mar. ’17) (IPE Mar. 15 (TS), 14 B.I.E)
Answer:
Lanthanoid contraction : The overall decrease in atomic and ionic radii from lanthanum to leutetium is observed in the lanthanoids. This phenomenon is called lanthanoid contraction. It is due to the fact that with every additional proton in the nucleus, the corresponding electron goes into a 4f-subshell. This is too diffused to screen the nucleus as effectively as the more localised inner shell. Hence, the attraction of the nucleus for the outermost electrons increases steadily with the atomic number.
Consequences of Lanthanoid Contraction : The important consequences of lanthanoid contraction are as follows :
i) Basic character of oxides and hydroxides : Due to the lanthanoid contraction, the covalent nature of La-OH bond increases and thus, the basic character of oxides and hydroxides decreases from La(OH)3 to Lu(OH)3.
ii) Similarity in the size of elements of second and third transition series : Because of lanthanoid contraction, elements which follow the third transition series are considerably smaller than would otherwise be expected. The normal size increases from Sc → Y → La and disappears after lanthanides. Thus, pairs of elements such as Zr/Hf, Nb/Ta and Mo/w are almost identical in size.
Due to almost similar size, such pairs have very similar properties which makes their separation difficult.
iii) Separation of lanthanoids : Due to lanthanoid contraction, there is a difference in some properties of lanthanoid like solubility, degree of hydration and complex formation. These difference enable the separation of lanthanoids by ion exchange method.
Question 3.
Explain Werner’s theory of coordination compounds with suitable examples. (Mar. ’14) (TS Mar. ’15) (IPE – May. 2015 (TS), ’14 B.I.E)
Answer:
Werner’s theory :
Postulates :
1) Every complex compound has a central metal atom (or) ion.
2) The central metal shows two types of valencies namely primary valency and secondary valency.
A) Primary valency : The primary valency is numerically equal to the oxidation state of the metal. Species or groups bound by primary valencies undergo complete ionization. These valencies are identical with ionic bonds and are non-directional. These valencies are represented by discontinuous lines (….)
Eg. : CoCl3 contains Co3+ and 3Cl– ions. There are three Primary Valencies or three ionic bonds.
B) Secondary Valency : Each metal has a characteristic number of Secondary Valencies. They are directed in space around the central metal.
The number of Secondary Valencies is called Coordination number (C.N.) of the metal. These valencies are directional in nature.
For example in CoCl3. 6NH3
Three Cl– ions are held by Primary Valencies and 6NH3 molecules are held by Secondary Valencies. In CuSO4.4NH3 complex \(\mathrm{SO}_4^{2-}\) rion is held by two Primary Valencies and 4NH3 molecules are held by Secondary Valencies.
3) Some negative ligands, depending upon the complex, may satisfy both primary and secondary valencies. Such ligands, in a complex, which satisfy both primary as well as secondary valencies do not ionize.
4) The primary valency of a metal is known as its outer sphere of attraction or ionizable valency while the Secondary valencies are known as the inner sphere of attraction or coordination sphere. Groups bound by secondary valencies do not undergo ionization in the complex.
Example to Clarify Werner’s Theory
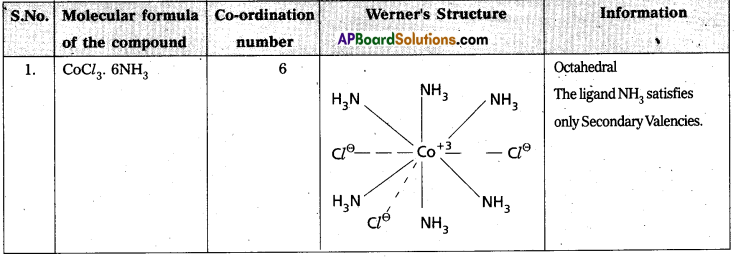
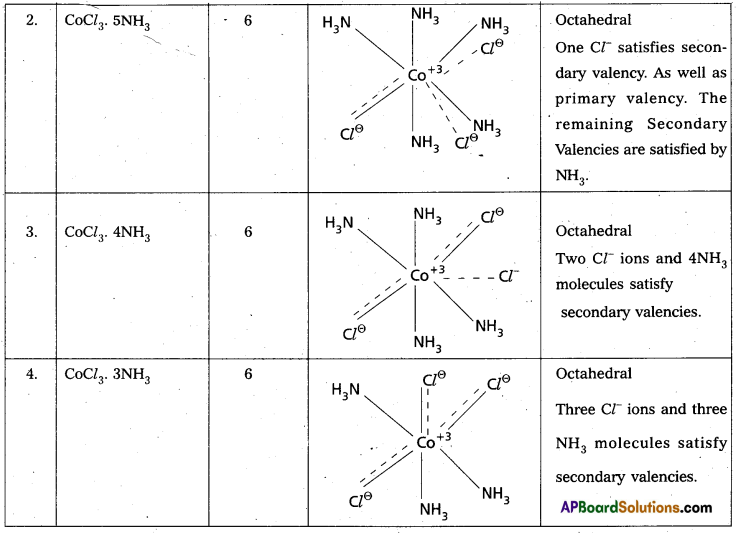
![]()
Question 4.
Using IUPAC norms write the formulas for the following.
- Tetrahydroxozincate (II)
- Hexaaminecobalt (III) sulphate
- Potassium tetrachloropalladate (II) and
- Potassium tri(oxalato) chromate (III) (IPE – May. 2015 (AP, TS), 14)
Answer:
- Tetrahydroxozincate (II) ion – [Zn(OH)4]-2
- Hexa amine cobalt (III) sulphate – [Co(NH3)6]2 (SO4)3
- Potassium tetrachioro palladate (II) – K2[PdCl4]
- Potassium tri(oxalato) chromate (III) – K3[Cr(C2O4)3]
Question 5.
What are homoleptic and heteroleptic complexes ? Give one example for each.
Answer:
Homoleptic complexes : These are the complexes in which a metal is bound by only ore kind of ligands, eg. : [Co(NH3)6]3+
Heteroleptic complexes: These are the complexes in which a metal is bound by more than one kind of ligands, eg. : [Co(NH3)4Cl2]+
Question 6.
Give the geometrical shapes of the following complex entities
- [CO(NH3)6]3+
- [Ni(CO)4]
- [Pt Cl4]2- and
- [Fe(CN)6]4-.
Answer:
- Geometrical shape of [CO(NH3)6]3+ is octahedral.
- Geometrical shape of [Ni(CO)4] is tetrahedral.
- Geometrical shape of [PtCl4]2- is square planar.
- Geometrical shape of [Fe(CN)6]-4 is octahedral.
Question 7.
Using IUPAC norms write the systematic names of the following.
- K4[Fe(CN)6]
- [Cu(NH3)4]SO4
- [Ti (H2O)6]3+ and
- [NiCl4]2-.
Answer:
- K4[Fe(CN)6] – Potassium hexa cyanao ferrate (II)
- [Cu(NH3)4]SO4 – Tetra amine copper (II) sulphate
- [Ti (H2O)6]3+ and – Hexa aquo titanium (III) ion
- [NiCl4]2- – Tetra chloro nickelate (II) ion
Question 8.
Explain the terms
(i) Ligand
(ii) Coordination number
(iii) Coordination entity
(iv) Central metaf atom/ion.
Answer:
i) Ligand : The ions, or molecules bound to the central atom/ion in the coordination entity are called ligands. These may be
a) simple ions such as Cl–
b) small molecules such as H2O or NH3
c) large molecules such as H2N.CH2CH2NH2 or N(CH2CH2NH2)3 or even
d) macro-molecules, such as proteins.
On the basis of the numberof donor atoms available for coordination, the ligands can be classified as :
a) Unidentate : One donor atom, Eg. ![]() etc.
etc.
b) Bidentate : Two donor atoms, Eg.: H2NCH2CH2NH2
(ethane-1, 2-diamine or en), C2\(\mathrm{O}_4^{2-}\) (oxalate), etc.
c) Polydentate : More than two donor atoms, Eg. : N(CH2CH2NH2)3 EDTA, etc.
ii) Coordination number : The coordination number (CN) of metal ion in a complex can be defined as the number of ligands or donor atoms to which the metal is directly bonded.
Eg : In [Ptcl6]2-, CN of Pt = 6, In [Ni(NH3)4]2+, CN of Ni = 4.
iii) Coordination entity: A central metal atoms or ions bonded to a fixed number of molecules or ions (ligands) is known as coordination entity. For example [CoCl3(NH3)3]. Ni(CO)4], etc.
iv) Central metal atom/ion : In a coordination entity, the atom/ion to which a fixed no. of ions/groups are bound in a definite geometrical arrangement around it is called central metal atom or ion. Eg : K4[Fe(CN)6] ‘Fe’ is central metal.
Question 9.
Explain the terms
- Unidentate ligand
- Bidentate ligand
- Polydentate ligand and
- Ambidentate ligand giving one example for each.
Answer:
- Unidentate : The negative ion or neutral molecule having only one donor atom is called unidentate ligand
Eg : etc.
etc. - Bidentate (or didentate) : The ions or molecules having two donor atoms are called

- Polydentate ligands : Ligands having more than one donor atom in the coordinating group and are capable of forming two or more coordinate bonds with same central atom simultaneously are called poly dentate ligands. Eg : C2\(\mathrm{O}_4^{-2}\).
- Ambidentate: Ligand which can ligate through two different atoms is called ambidentate ligand. Eg : \(\mathrm{NO}_2^{-}\), \(\mathrm{SCN}^{-}\) ions. \(\mathrm{NO}_2^{-}\) ion can coordinate either through nitrogen or through oxygen to a central metal atom/ion. Similarly, \(\mathrm{SCN}^{-}\) ion can coordinate through the sulphur or nitrogen atom.
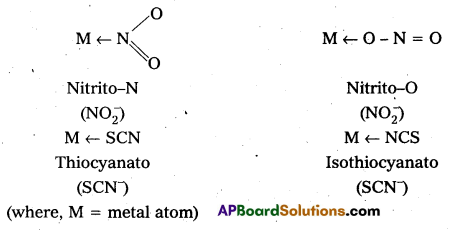
![]()
Question 10.
Explain the colour and para magnetic property of transition elements.
Answer:
Transition elements having unpaired electrons in d-orbitals are coloured. The colour is due to d-d transitions of electrons. Electrons are excited from lower set of d-orditals to higher set of d- orbitals by absorbing energy from visible light and emits complementary colour which is the colour of the ion. Transition elements are ions having atleast one unpaired electron in d-orbitals are para magnetic. The extent of para magnetic property is expressed in terms of magnetic moment (μ). μ = \(\sqrt{n(n+2)}\) where n is number of unpaired electrons present in d- orbitals.
Question 11.
Explain different types of isomerism exhibited by Co-ordination compounds, giving suitable examples.
Answer:
Isomerism in Co-ordination compounds : Isomers are compounds that have the same chemical formula but different arrangement of atoms. Two principal types of isomerism are known among co-ordination compounds namely stereo isomerism and structural isomerism.
a) Stereoisomerim : Stereoisomerism is a form of isomerism in which two substances have the same composition and structure but differ in the relative spatial positions of the ligands. This can be sub divided into two classes namely.
i) Geometrical isomerism and
ii) optical isomerism
b) Structural isomerism :
i) Linkage isomerism
ii) Co-ordination isomerism
iii) Ionisation isomerism
iv) Hydrate isomerism
a) (i) Geometrical isomerism :
- Geometrical isomerism arises in Co-ordination complexes due to different possible geometric arrangements of the ligands.
- This isomerism found in complexes with Co-ordination numbers 4 and 6.
- In a square planar complex of formula [Mx2L2] the two ligands may be arranged in adjacent to each other in a cis isomer (or) opposite to each other in a trans isomer.
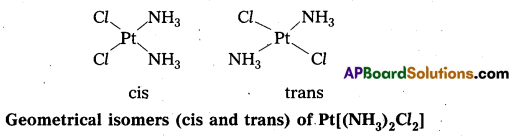
- Square planar complex of type [MAB XL] shows three isomers two-cis and one trans. (A, B, X, L are unidentate ligands is square planar complex).
- Geometrical isomerism is not possible in tetrahedral geometry.
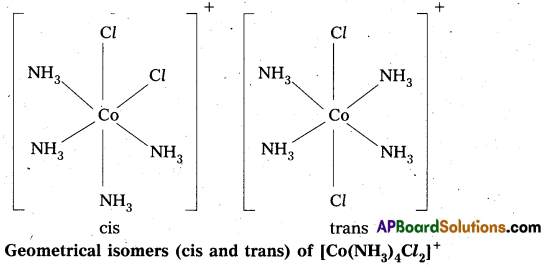
- In octahedral complex of formula [MX2L4] in which two ligands X may be oriented cis or trans to each other.
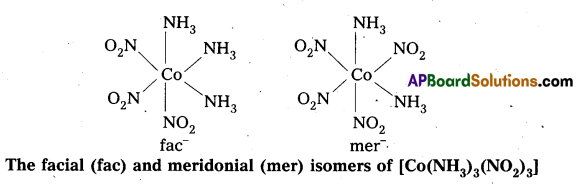
- Another type of geometrical isomerism occurs in octahedral Co-ordination compounds of type [Ma3b3] if three donor atoms of the same ligands occupy adjacent positions at the comers of an octahedral face then it is facial (fac) isomer. When the positions occupied are around the meridian of the octahedran then it is meridonial (mer) isomer.
a(ii) Optical isomerism : Optical isomerism arises when two isomers of a compound exist such that one isomer is a mirror image of the other isomer. Such isomers are called optical isomers or enantiomers. The molecules or ions that cannot be superimposed are called chiral.
The two forms are called dextro (d) and laevo (l) depending upon the direction they rotate the plane of polarised light in a polarimeter (d rotates to the right, l to the left). Optical isomerism is common in octahedral complexes involving bidentate ligands.
b(i) Linkage isomerism: Linkage isomerism arises in Co-ordination compound containing ambidentate ligand. A simple example is provided by complexes containing the thiocyanate ligand-NCS–, which may bind through the nitrogen to give M-NCS or through sulphur to give M-SCN.
eg. : [Mn(CO)5SCN] and [Mn(CO)5NCS]
(ii) Co-ordinate isomerism : This type isomerism arises from the interchange of ligands between cationic and anionic entities of different metal ions present in a complex.
eg. : [CO(NH3)6] [Cr(CN)6] and [Co(CN)6] [Cr(NH3)6]
(iii) Ionisation isomerism: This form of isomerism arises when the counter ion in a complex salt is itself a potential ligand and can displace a ligand which can then become the counter ion.
eg. : [Co(NH3)5SO4] Br and [Co(NH3)5Br]SO4
(iv) Hydrate isomerism : This form of isomerism is known as ‘hydrate isomerism since water is involved as a solvent. Hydrate isomers differ by whether or not a hydrate molecule is directly bonded to the metal ion or merely present as free solvent molecules in the crystal lattice.
Question 12.
What is meant by chelate effect ? Give example.
Answer:
When a didentate or a polydentate ligand contains donor atoms positioned in such a way that when they coordinate with the central metql ion, a 5-or 6-membered ring is formed, the effect is known as chelate effect. Example
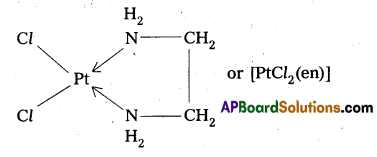
Question 13.
Discuss the nature of bonding and magnetic behaviour in the following co-ordination entities on the basis of valence bond theory.
(i) [Fe(CN)6]4-
(ii) [FeF6]3-
(iii) [Co(C2O4)3]3- and
(iv) [CoF6]3-
Answer:
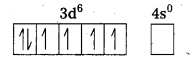
i) [Fe(CN)6]4- : In this complex Fe is present as Fe2+.
Fe = [Ar] 3d64s2
Outer configuration of Fe2+ = 3d64s0
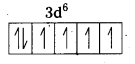
CN– being strong field ligand, pair up the unpaired d electrons Thus, two 3d-orbital are now available for CN– ions.
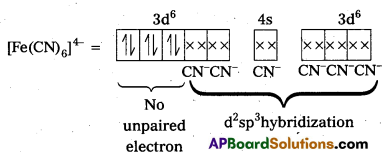
Since, all the electrons are paired, the complex is diamagnetic. Moreover (n – 1) d-orbitals are involved in bonding, so, it is an inner orbital or low spin complex.
ii) [FeF6]3- : In this complex, the oxidation state of Fe is + 3.

F- is not a strong field ligand. It is a weak field ligand, so no pairing occurs. Thus, 3d- orbitals are not available to take part in bonding.
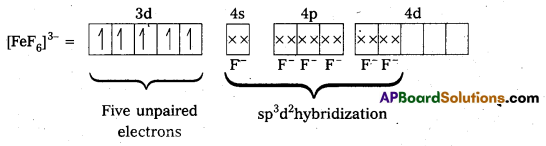
Because of the presence of five unpaired electrons, the complex is paramagnetic. Moreover, nd-orbitals are involved in bonding, so it is an outer orbital or high spin complex.
iii) [Co(C2O4)3]3- : In this complex, the oxidation state of Co is + 3.
Outer configuration of Co = 3d7 4s2
Co3+ = 3d64s0
Oxalate ion being a strong field ligand pair up the 3d electrons, thus two out of the five 3d-orbitals are available for oxalate ions.
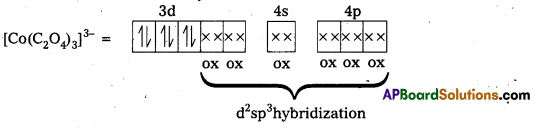
Since, all the electrons are paired, this complex is diamagnetic. It is an inner orbital complex because of the involvement of (n – 1) d-orbital for bonding,
iv) [CoF6]3- : In this complex, Co is present as Co3+
Outer configuration of Co3+ = 3d6 4s0
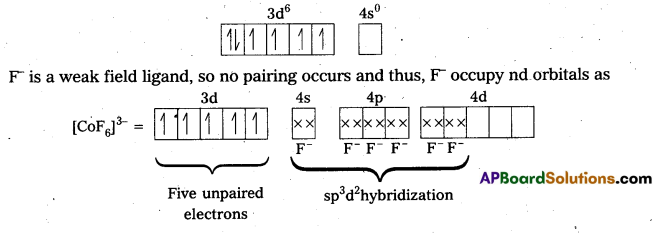
Because of the presence of four unpaired electrons, the complex is paramagnetic. Since, nd orbitals take part in bonding, it is an outer orbital complex or high spin complex.
![]()
Question 14.
What is spectrochemical series ? Explain the difference between a weak field ligand and a strong field ligand.
Answer:
The arrangement of ligands in order of their increasing field strengths, i.e., increasing crystal field splitting energy (CFSE) values is called spectrochemical series.
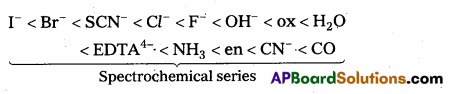
The ligands with a small value of CFSE (Δ0) are called weak field ligands. For such ligands Δ0 < P where P is the pairing energy. Whereas the ligands with a large value of CFSE are called strong field ligands. In case of such ligands Δ0 > R
When ligands approach a transition metal ion, the d-orbitals split into two sets (t2g and eg), one with lower energy and the other with higher energy. The difference of energy between the two sets of orbitals is called crystal field splitting energy, Δ0 for octahedral field and Δt for tetrahedral field.
If Δ0 < P (pairing energy), the 4th electron enters one of the eg orbitals giving the configuration \(t_{2 g}^3 e_g^1\) thereby forming high spin complex. Such ligands for which Δ0 < P are known as weak field ligands.
If Δ0 > P, the 4th electron pairs up in one of the t2g orbitals giving the configuration \(t_{2 g}^3 e_g^1\), thus forming low spin complexes. Such ligands for which Δ0 > P are called strong field ligands.
Question 15.
Explain the terms
(i) Ligand
(ii) Coordination number
(iii) Coordination entity
(iv) Central metal atom/ion.
Answer:
i) Ligand : The ions or molecules bound to the central atom/ion in the coordination entity are called ligands. These may be
a) simple ions such as Cl–
b) small molecules such as H2O or NH3
c) large molecules such as H2NCH2CH2NH2 or N(CH2CH2NH2)3 or even
d) macro-molecules, speh as proteins.
On the basis of the number of donor atoms available for coordination, the ligands can be classified as :
a) Unidentate : One donor atom, Eg.: ![]() etc.
etc.
b) Bidentate : Two donor atoms, Eg.: H2NCH2CH2NH2
(ethane-1, 2-diamine or en), C2\(\mathrm{C}_2 \mathrm{O}_4^{2-}\) (oxalate), etc.
c) Polydentate: More than, two donor atoms, Eg.: N(CH2CH2NH2)3 EDTA etc.
ii) Coordination number: The coordination number (CN) of metal ion in a complex can be defined as thê number of ligands or donor atoms to which the metal is directly bonded.
Eg: In [PtCl6]2-, CN of Pt = 6, In [Ni(NH3)4]2+, CN of Ni = 4.
iii) Coordination entity : A central metal atoms or ion bonded to a fixed number of molecules or ions (ligands) is known as coordination entity. For example [COCl3(NH3)3]. Ni(CO)4], etc.
iv) Central metal atom/ion: In a coordination entity, the atom/ion to which a fixed no. of ions/groups are bound in a definite geometrical arrangement around it is called central metal atom or ion. Eg: K4[Fe(CN)6] ‘Fe’ is central metal.