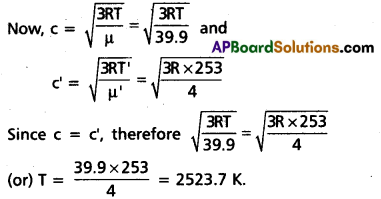
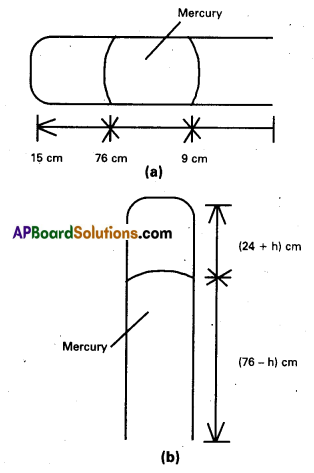
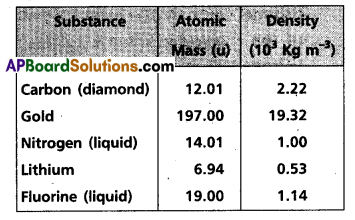
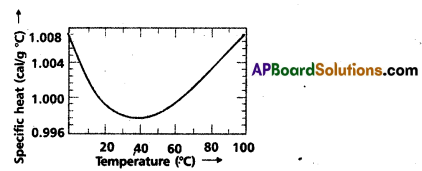
Andhra Pradesh BIEAP AP Inter 2nd Year Civics Study Material 8th Lesson State Judiciary Textbook Questions and Answers.
AP Inter 2nd Year Civics Study Material 8th Lesson State Judiciary
Long Answer Questions
Question 1.
Explain the powers and functions of the High Court.
Answer:
The constitution of India provides for a High Court for each state. But the 7th Amendment Act, of 1956 authorized the Parliament to establish a common High Court for two or more states and a Union Territory.
Articles 214 to 231 in Part-VI of the constitution deal with the organization, qualifications, appointment, independence, jurisdiction, powers, and procedures, etc., of the High Court.
Composition :
Every High Court consists of a Chief Justice and such other judges as the President may from time to time deem it necessary to appoint.
Appointment of Judges :
The Chief Justice of High Court is appointed by the President after consultation with the Chief Justice of India and the Governor of the concerned state. The other judges of High Court are appointed by the President with the consultation of the Chief Justice of High Court of the concerned State. In case of a common High Court for two or more States, the Governors of concerned States are cpnsulted by the President. ,
Qualifications of Judges :
A person to be appointed as a judge of High Court should possess the following qualifications.
a) He should be a citizen of India.
b) He should have held judicial office in the territory of India for atleast 10 years, or
c) He should have been an advocate of a High Court or of two or more such courts for 10 years period. However, the Constitution has not prescribed a minimum age for appointment as a judge of a High Court.
Salaries and Allowances :
The salaries, allowances, privileges, leave and pension of the judges of a High Court are determined by the Parliament from time to time. The Judge of a High Court gets a salary of ₹ 80,000/-per month and the Chief Justice gets ₹ 90,000/-. They are also entitled to get other allowances and are provided with free accommodation and other facilities like medical, car, telephone etc.
The salaries and allowances cannot be reduced except under financial emergency. The salaries and allowances are drawn from the Consolidated Fund of the State. Their retired Chief Justice and other judges are entitled to 50% of their last drawn salary as monthly pension.
Tenure :
Every Judge of a High Court including Chief Justice holds office until he attains the age of 62 years. The Judges including the Chief Justice will take oath in the presence of the Governor of the concerned State. He can resign for his office when he desire so by writing to the President to that effect.
Method of removal:
A Judge of a High Court can.be removed by the President on the grounds of proved misbehaviour or incapacity. The method of removal of a Judge of the High Court shall be the same as that of a Judge of the Supreme Court.
Powers and Functions of the High Court:
The following are the powers and functions of the High Court.
1) Original Jurisdiction:
Every High Court in India has original jurisdiction in regard to matters of admiralty, will, marriage, divorce, company laws, contempt of court and certain revenue cases. Every High Court is empowered to issue directions, orders or writs for the enforcement of any of the Fundamental Rights. Every High Court is empowered to settle disputes relating to election of members of Parliament and State Legislatures.
Under Article 226, the High Court is empowered to issue writs for enforcing Fundamental Rights. The High Court issue writs like Habeas-corpus, Certiorari, Mandamus, Quo-warranto and Injunction,for protecting the Fundamental Rights of the Indian Citizens.
2) Appellate Jurisdiction :
Every High Court hears appeals against the judgement rdinate courts. The appellate Jurisdiction of the High Court extends to both riminal Cases.
a) Civil Cases :
An appeal to the High Court on the civil side is either a first appeal or a second appeal. In civil cases, appeal to the High Court lies from the decision of a District Court. Appeals can also be made from the subordinate courts directly provided the dispute involves a value of more than ₹ 5,00,000/- (or) a question of fact of law. When a court subordinate to the High Court decides an appeal differing from the decision of an inferior court, a second appeal can be made to the High Court.
b) Criminal Cases :
In Criminal cases, it hears the appeals in which the accused has been sentenced to more than seven years imprisonment by the Sessions Judge. All cases involving capital punishment awarded by the Sessions Court come to High Court as appeals. Its approval is necessary for the imposition of death sentences by the District Sessions Judge. It also hears cases involving the interpretation of the Constitution.
3) Court of Record :
The State High Court acts as a Court of Record. It records all its decisions and judgements. Such records are of great significance. They carry evidentary value. They are taken as Judicial precedents to the Judges and Advocates in legal matters.
4) Power of Judicial Review :
The State High Court possesses the power of judicial review like the Supreme Court. It is the power of High Court to examine the constitutionality of legislature enactments and executive orders of both the Central and State Governments. On examination, if they are found to bp violated of the Constitution (Ultra Vires), they can be declared as illegal, unconstitutional and invalid (null and void) by the High Court. Consequently, they cannot be enforced by the government.
5) Power of Certification :
High Court certifies certain cases which can go to the Supreme Court. That appeals which go to Supreme Court depend up on the issue of a certificate by the High Court.
6) Advisory functions :
The High Court is consulted by the State Governor in the matters of appointment, posting and promotion of District Judges and in the appointment of personnel to the Judicial Services of the State (Other than District Judges). It deals with the matters of posting, promotion, grant of leave, transfers and discipline of the members of the Judicial Service of the State. It also, renders advice to the subordinate courts in the matters of public interest or of legal importance.
7) Administrative functions :
The High Court exercises certain administrative functions within its territorial jurisdictions.
- Under Article 227, every High Court has the power of supervision over all Courts and Tribunals functioning in its territorial jurisdiction except Military Courts or Tribunals in the State.
- It ensures the proper working of these courts. It exercises the power to make and issue general rules and regulations for securing the efficient working of the court.
- The High Court can transfer any case from one court to another court under Article 228 and can even transfer the case itself and decide the same.
- The High Court has the power to investigate or enquire into the records or other connected documents for any court subordinate to it.
- It appoints its administrative staff and determines the salaries and allowances and other conditions of the personnel working in subordinate courts.
- It is empowered to withdraw any case involving the interpretation of the constituion and dispose of the case itself.
- The High Court is the Highest Court of Justice in the State. All other Courts and Tribunals (except Military Courts and Tribunals) in State function under the direct supervisions and control of the State High Court.
Other functions :
a) The High Court acts as the District Court where its head quarters are located.
b) The Chief Justice of the High Court acts as the Governor on the direction of the President tentatively whenever the vacancy arises in that office.
c) The High Court can admit Public Interest Litigation like the Supreme Court of India.
![]()
Question 2.
Write an essay on the District Level Courts.
Answer:
The state judiciary consists of a High Court and a hierarchy of Subordinate Courts also known as District Courts. The District Courts play an important role in judicial administration at the district level. The courts consist of District Judge and other Judges. They fullfill their obligations at the District, Town and Major Village levels. They hear civil and criminal cases. They are subject to the authority and control of the State government in administrative matters and to the State High Court in judicial matters.
There are two types of subordinate courts in a State namely :
- Civil Courts and
- Criminal Courts.
1) Civil Courts :
The Civil Courts deal with civil suits regarding the matter like marriages, divorce, inheritance, business etc. There will be District Civil Courts at the District Level. The District Judge acts as its head. He exercises control and supervision over other civil courts in the district.
There are some senior civil judge courts below the rank of the district civil courts. There are some other junior civil judge courts in addition senior civil judge court. Judicial officers of subordinate courts are given here under :
- Principal District Judge
- Family Court Judge
- SC & ST Act Court Judge
- Senior Civil Court Judge
- Junior Civil Court Judge
The Principal District Court admits the cases pertaining to an amount of Rupees 10 Lakhs and above worth of poverty and deliver the judgements. The Principal District Judge is appointed through direct as well as indirect recruitment (By promotion).
The Family Courts are presided by judicial officers of the cadre of District Judges. This court takes up cases under Hindu Marriage Act relating to divorce, ordering interim maintenance, ordering custody of children etc. In order to protect Scheduled Caste and Scheduled Tribes rights and to implement SC & ST Act strictly, there is a court for the entire district.
There are some courts namely Senior Civil Judge Courts which deal with the cases of property worth rupees above one lakh and below 10 lakhs and deliver the judgements.
Cases pertaining to property worth below one lakh will be taken up by Junior Civil Judge Court and the Judgements are delivered. There are some Nyaya Panchayats, Grama Kacheries, Adalati Panchayats and so on at the lowest level in the district to deal with local legal issues.
2) Criminal Courts :
The Sessions Court is the highest criminal court in the district. The Sessions court acts as the superior court at the district level in handling the criminal matters. The Sessions Judge delivers judgements according to the provisions mentioned in the Indian penal code and the criminal procedure code. The following judges deal with at the district level.
They are :
- District Sessions Judge
- Senior Assistant Sessions Judge
- Junior Civil Judge
- Special Judicial Magistrate
The Principal District Judge will act as District Sessions Judge, who deals with the cases relating to murder and motor vehicles act violation cases and delivers the judgement and imposes life imprisonment or death sentences which are to be confirmed by the State High Court. The Senior Assistant Sessions Judge will impose an imprisonment of five to seven years, depending on the nature of the Case.
If there is a Junior Civil Judge Court for the entire town the court acts as a civil as well as a criminal court and take up the cases and deliver judgement and impose imprisonment below three years. There are Second Class Magistrate Courts which deliver the judgement by imposing fine up to rupees five hundred or a sentence of one year or both.
Special Judicial Magistrate Courts will be established in every town which takes up petty cases and deliver the judgement by imposing fines below ₹ 500/- and impose imprisonment below six months.
Short Answer Questions
Question 1.
Explain briefly the composition of High Court.
Answer:
The Constitution of India provides for a High Court for each state. But the 7th Amendment Act, 1956 authorised the Parliament to establish a common High Court for two or more states and a Union Territory. *
Composition :
Every High Court shall consist of a Chief Justice and some other Judges. The President of India may appoint them from time to time. Besides, the President has the power to appoint Additional Judges for a temporary period not exceeding two years as an acting Judge, where a permanent Judge of a High Court is temporarily absent or unable to perform his duties.
Such judges hold office until the permanent Judge resumes his office. The number of Judges varies from 5 in Gauhati High Court to 48 in the Allahabad High Court. Our Constitution does not specify the exact strength of High Court judges and leaves it to the discretion of the President. Accordingly, the President determines the strength of a High Court from time to time depending upon its workload.
Question 2.
Write any two powers and functions of the State High Court. [Mar. 18, 16]
Answer:
The following are the two powers and functions of the High Court.
1) Original Jurisdiction:
Every High Court in India has original jurisdiction in regard to matters of admiralty, will, marriage, divorce, company laws, contempt of court and certain revenue cases. Every High Court is empowered to issue directions, orders or writs for the enforcement of any of the Fundamental Rights. Every High Court is empowered to settle disputes relating to election of members of Parliament and State Legislatures. The High Courts of Bombay, Calcutta and Madras possess original jurisdiction in Civil as well as Criminal cases arising within the presidency towns.
They are authorized to hear Civil Cases involving property of the value of ₹ 20,000/- or more. They enjoy exclusive privileges and authority in this regard. In fact this power of High Court was m vogue before independence. It has been retained in the new Constitution. The other High Courts also enjoy the same jurisdiction as was available to them before independence.
Under Article 226, the High Courts is empowered to issue writs for enforcing Fundamental Rights. The High Court issue writs like Habeas-corpus, Certiorari, Mandamus, Quo-warranto and Injunction for protecting the Fundamental Rights of the India Citizens.
2) Appellate Jurisdiction :
Every High Court hears appeals against the judgement of the subordinate courts. The appellate Jurisdiction of the High Court extends to both Civil and Criminal Cases.
a) Civil Cases :
An appeal to the High Court on the civil side is either a first appeal or a second appeal. In civil cases, appeal to the High Court lies from the decision of a District Court. Appeals can also be made from the subordinate courts directly provided the dispute involves a value of more than ₹ 5,00,000/- (or) a question of fact of law. When a court subordinate to the High Court decides an appeal differing from the decision of an inferior court, a second appeal can be made to the High Court.
b) Criminal Cases :
In Criminal cases, it hears the appeals in which the accused has been sentenced to more than seven years imprisonment by the Sessions Judge. All cases involving capital punishment awarded by the Sessions Court come to High Court as appeals. Its approval is necessary for the imposition of death sentences by the District Sessions Judge. It also hears cases involving the interpretation of the Constitution.
Question 3.
Explain the Administrative functions of the High Court
Answer:
The High Court exercises certain administrative functions within its territorial jurisdictions.
- Under Article 227, every High Court has the power of supervision over all Courts and Tribunals functioning in its territorial jurisdiction except Military Courts or Tribunals in the State.
- It ensures the proper working of these courts. It exercises the power to make and issue general rules and regulations for securing the efficient working of the court.
- The High Court can transfer any case from one court to another court under Article 228 and can even transfer the case itself and decide the same.
- The High Court has the power to investigate or enquire into the records or other connected documents of any court subordinate to it.
- It appoints its administrative staff and determines the salaries and allowances and other conditions of the personnel working in subordinate courts.
- It is empowered to withdraw any case involving the interpretation of the Constitution and dispose of the case itself.
- The High Court is the Highest Court of Justice in the State. All other Courts and Tribunals (except Military Courts and Tribunals) in State function under the direct supervision and control of the State High Court.
![]()
Question 4.
Explain the powers and functions of District Court.
Answer:
There will be District Civil Courts at the District Level. The District Judge acts as its head. He exercises control and supervision over other civil courts in the district.
There are some senior civil judge courts below the rank of the district civil courts. There are some other junior civil judge courts in addition senior civil judge courts. Judicial officers of subordinate courts are given here under :
- Principal District Judge
- Family Court Judge
- SC & ST Act Court Judge
- Senior Civil Court Judge
- Junior Civil Court Judge
The Principal District Court admits the cases pertaining to an amount of Rupees 10 Lakhs and above worth of property and deliver the judgements. The Principal District Judge is appointed through direct as well as indirect recruitment (By promotion).
The Family Courts are presided by judicial officers of the cadre of District Judges. This court takes up cases under Hindu Marriage Act relating to divorce, ordering interim maintenance, ordering custody of children etc. In order to protect Scheduled Caste and Scheduled Tribes rights and to implement SC & ST Act strictly, there is a court for the entire district. .
There are some courts namely Senior Civil Judge Courts which deal with the cases of property worth rupees above one lakh and below 10 lakhs and deliver the judgements.
Cases pertaining to property worth below one lakh will be taken up by Junior Civil Judge Court and the Judgements are delivered. There are some Nyaya Panchayats, Grama Kacheries, Adalati Panchayats and so on at the lowest level in the district to deal with local legal issues.
Question 5.
Discuss the powers and functions of State Advocate General. [Mar. 17]
Answer:
Every State in Indian Union shall have an Advocate General, an official corresponding to the Attorney-General of India. He performs similar functions for the State that of the Attorney-General of India. He is the highest law officer in the State.
Appointment:
The Advocate General is appointed by the Governor of the State under the Article 165 of the Constitution. A person to be appointed as Advocate General must possess the following qualifications.
- He should be a citizen of India.
- He must have held a judicial office for ten years or an advocate of a High Court for ten years.
- He must be a person who is qualified to be appointed a judge of a High Court.
Tenure and Removal :
The Constitution of India did not mention the tenture of Advocate General. Further, the Constitution does not contain the procedure and grounds for his removal. He holds office during the pleasure of the Governor. He may be removed by the Governor at any time. He may also quit his office by submitting his resignation to the Governor. Conventionally, he resigns when the government resigns or is replaced, as he is appointed on the advice of the government.
Salary :
His remuneration is not fixed by the Constitution. He receives such remuneration as the Governor may decide from time to time.
Powers and functions :
As the highest law officer of the State Government, he exercises the following powers and functions.
- He advises the State Government upon such legal matters which are referred to him by the Governor.
- He performs such other duties of a legal character that are assigned to him by the Governor.
- He discharges the functions and conferred on him by the Constitution.
- He appeared before any court of law within the State.
- He has a right to speak and to take part as member in the proceedings of the house (s) but no right to vote.
- He can also attend any of the Standing Committee meetings of State Legislature.
Very Short Answer Questions
Question 1.
Appointment of High Court Judges. [Mar. 18, 17]
Answer:
The Chief Justice of High Court is appointed by the President after consultation with the Chief Justice of India and the Governor of the concerned State. The other judges of High Court are appointed by the President with the consultation of the Chief Justice of High Court of the concerned State. In case of a common High Court for two or more States, the Governors of concerned States are consulted by the President.
Question 2.
Qualifications of High Court Judges. [Mar. 16]
Answer:
A person to be appointed as judge of High Court should possess the following qualifications.
a) He should be a citizen of India.
b) He should have held a judicial office in the territory of India at least 10 years, or
c) He should have been an advocate of a High Court or of two or more such courts for 10. years period. However, the constitution has not prescribed a minimum age for appointment as a judge of a High Court.
Question 3.
High Court as a Court of Record.
Answer:
The State High Court acts as a court of Record. It records all its decisions and judgements. Such records are of great significance. They carry evidentary value. They are taken as Judicial precedents to the judges and Advocates in legal matters.
![]()
Question 4.
Advisory functions of High Court.
Answer:
The High Court is consulted by the State Governor in the matters of appointment, posting and promotion of District Judges and in the appointment of personnel to the Judicial Services of the State (Other than District Judges). It deals with the matters of posting, promotion, grant of leave, transfers and discipline of the members of the Judicial Service of the State. It also renders advice to the subordinate courts in the matters of public interest or of legal importance.