Practice the AP 8th Class Physical Science Bits with Answers Chapter 5 Metals and Non-Metals on a regular basis so that you can attempt exams with utmost confidence.
AP State Syllabus 8th Class Physical Science Bits 5th Lesson Metals and Non-Metals with Answers
Choose the correct answer.
Question 1.
Which one of the following is not a non-metal?
A) Iron
B) Copper
C) Aluminium
D) Sulphur
Answer:
D) Sulphur
Question 2.
……….. is the liquid metal.
A) Iodine
B) Gold
C) Silver
D) Mercury
Answer:
D) Mercury
![]()
Question 3.
The property of a material to produce a particular sound when dropped down is
A) sonority
B) malleability
C) ductility
D) none of these
Answer:
A) sonority
Question 4.
The property of drawing a material to make fine wires is called
A) malleability
B) ductility
C) conductivity
D) none of these
Answer:
B) ductility
Question 5.
Magnesium oxide is
A) basic oxide
B) acidic oxide
C) neutral oxide
D) none of the above
Answer:
A) basic oxide
Question 6.
Sulphur dioxide is a
A) Basic oxide
B) Acidic oxide
C) Neutral oxide
D) Dual Natural oxide
Answer:
B) Acidic oxide
![]()
Question 7.
Generally non-metallic oxides are ……….. in nature.
A) Basic
B) Acidic
C) Neutral
D) Dual nature
Answer:
B) Acidic
Question 8.
The property of changing the metals into sheets is
A) Malleability
B) Ductility
C) Conductivity of heat
D) Electric conductivity
Answer:
A) Malleability
Question 9.
The metal which is in liquid state is
A) Gold
B) Mercury
C) Copper
D) Iron
Answer:
B) Mercury
Question 10.
Non-metal which is lustrous
A) iodine
B) bromine
C) chlorine
D) sulphate
Answer:
A) iodine
![]()
Question 11.
The non-metal which is in liquid state
A) Chlorine
B) Iodine
C) Bromine
D) Sulphur
Answer:
C) Bromine
Question 12.
Calcium oxide is
A) basic oxide
B) acidic oxide
C) neutral oxide
D) amphoteric oxide
Answer:
A) basic oxide
Question 13.
………….. do not react with acids.
A) Metals
B) Non-metals
C) Metalloids
D) None of these
Answer:
B) Non-metals
Question 14.
………… are good conductors of heat and electricity.
A) Metalloids
B) Non-metals
C) Metals
D) None of these
Answer:
C) Metals
![]()
Question 15.
Metals have shiny surface. This property is known as
A) malleability
B) ductility
C) sonorous
D) lustrous
Answer:
D) lustrous
Question 16.
The property of materials which can be beaten into thin sheets is called
A) malleability
B) ductility
C) sonorous
D) lustrous
Answer:
A) malleability
Question 17.
Metals react with acids and liberate gas
A) oxygen
B) hydrogen
C) chlorine
D) nitorgen
Answer:
B) hydrogen
Question 18.
Oxides of non-metals are usually in …………. nature.
A) acidic
B) basic
C) neutral
D) none
Answer:
A) acidic
![]()
Question 19.
Oxides of metals are usually in ………… nature.
A) acidic
B) basic
C) neutral
D) amphoteric
Answer:
B) basic
Question 20.
The non-metal present in anions ……………
A) chlorine
B) sulphur
C) oxygen
D) hydrogen
Answer:
B) sulphur
Question 21.
Most of metals exist in …………. state.
A) solid
B) liquid
C) gas
D) none of these
Answer:
A) solid
Question 22.
………… properties better judge a material is metal or non-metal.
A) Physical
B) Chemical
C) Cannot say
D) None of these
Answer:
B) Chemical
![]()
Question 23.
Sodium is stored in …………
A) water
B) air
C) kerosene
D) none of these
Answer:
C) kerosene
Question 24.
Gold and platinum are called ………… metals.
A) inert
B) noble
C) rare
D) none of these
Answer:
B) noble
Question 25.
The essential non-metal for all living things is …………..
A) Hydrogen
B) Oxygen
C) Chlorine
D) Sulphur
Answer:
B) Oxygen
Question 26.
Best conductor of electricity is
A) silver
B) gold
C) copper
D) aluminium
Answer:
A) silver
![]()
Question 27.
Among which is does not react with air?
A) Sodium
B) Potassium
C) Cesium
D) Gold
Answer:
D) Gold
Question 28.
The non-metal added in gun powder is
A) hydrogen
B) chlorine
C) sulphur
D) none of these
Answer:
C) sulphur
Question 29.
Which metal is mainly used in preparation of machinery?
A) silver
B) gold
C) copper
D) iron
Answer:
D) iron
Question 30.
Which of these metals is main component in all the fuels?
A) Copper
B) Zinc
C) Aluminium
D) Carbon
Answer:
D) Carbon
![]()
Question 31.
Electric wires are mainly prepared with …………. metal.
A) copper
B) zinc
C) sodium
D) potassium
Answer:
A) copper
Question 32.
Assertion (A): Mercury is not a metal.
Reason (R): Mercury does not show sonorous, malleability and ductility.
A) A and R are true
B) A and R are false
C) A is true, but R is false
D) A is false, but R is true
Answer:
D) A is false, but R is true
Question 33.
Veni: All metals are shines.
Sana: All shines are metals.
A) Veni is correct but Sana is wrong
B) Veni is wrong but Sana is correct
C) Both are correct
D) Both are wrong
Answer:
A) Veni is correct but Sana is wrong
Question 34.
Assertion (A): Bases are prepared with oxides of metals.
Reason (R): Bases changes red litmus paper into blue.
A) A and R are true R does not support A
B) A and R are true R supports A
C) ‘A’ is true but ‘R’ is false
D) A is false but ‘R’ is true
Answer:
A) A and R are true R does not support A
![]()
Question 35.
Find the correct one.
A) Oxides of non – metals are usually acidic in nature
B) Oxides of metals are usually basic in nature
C) Both ‘A’ and ‘B’
D) Neither A’ nor ‘B’
Answer:
C) Both ‘A’ and ‘B’
Question 36.
What will happen if you use wooden bell in your school?
A) It rings with more intensity of sound
B) It does not ring
C) It does not vibrate while ringing
D) It rings with less intensity of sound
Answer:
D) It rings with less intensity of sound
Question 37.
Predict a metal, which is a metal but not has sonority.
A) Carbon
B) Mercury
C) Brass
D) Gold
Answer:
B) Mercury
Question 38.
How can you say plastic does not have property of malleability?
A) Plastic does not available in the form of thin sheets
B) Plastic does not available in the form of wires
C) Plastic cannot change into thin sheets by hammering
D) Above all
Answer:
C) Plastic cannot change into thin sheets by hammering
![]()
Question 39.
There is substance ‘X’. After burning, it is changed into powder. If we add water to the powder it acts as a base, then the ‘X’ may be
A) Mg (Magnesium)
B) C (Carbon)
C) O (Oxygen)
D) Gold
Answer:
A) Mg (Magnesium)
Question 40.
What happens if we add copper dust into Ferrous sulphate solution?
A) Copper displaces Ferrous
B) Copper does not displace Ferrous
C) We cannot say anything
D) Copper dissolves in Ferrous sulphate solution
Answer:
A) Copper displaces Ferrous
Question 41.
Guess the reason why does Ferrous (iron) does not displace Zinc from ZnSO4 (Zinc sulphate solution)
A) Ferrous is more reactive metal than Zinc
B) Zinc is more reactive metal than Ferrous
C) Ferrous and Zinc are non-metals
D) Ferrous and Zinc are metals
Answer:
B) Zinc is more reactive metal than Ferrous
Question 42.
Some metals reacts with acids to evolve gas-
A) Hydrogen
B) Oxygen
C) Carbon dioxide
D) Nitrogen
Answer:
A) Hydrogen
![]()
Question 43.
………….. generally do not react with water.
A) Metals
B) Non-metals
C) Metalloids
D) None of these
Answer:
B) Non-metals
Question 44.
………… metal get green coating when exposed to air.
A) Silver
B) Copper
C) Gold
D) Aluminium
Answer:
B) Copper
Question 45.
………….. do not react with air.
A) Gold
B) Silver
C) Copper
D) Iron
Answer:
A) Gold
Question 46.
Iron is ………… reactive than copper.
A) less
B) more
C) equal
D) none of these
Answer:
B) more
![]()
Question 47.
………….. metal gets rust when exposed to air.
A) Gold
B) Platinum
C) Zinc
D) Iron
Answer:
D) Iron
Question 48.
The gas which produce pop sound
A) oxygen
B) hydrogen
C) chlorine
D) nitrogen
Answer:
B) hydrogen
Question 49.
When zinc granules are added to copper sulphate is deposited at the bottom.
A) copper
B) zinc
C) sulphur
D) oxygen
Answer:
A) copper
Question 50.
When exposed to air silver objects and jewellery become
A) white
B) green
C) blue
D) black
Answer:
D) black
![]()
Question 51.
Choose for correct matching.
Group – A — Group – B
1. Metallic — a) does not react oxides with air
2. Non-metallic — b) eggs oxides
3. Iron — c) basic
4. Gold — d) get rusted
5. Sulphur — e) acidic
A) 1 – c, 2 – e, 3 – d, 4 – a, 5 – b
B) 1 – a, 2 – b, 3 – c, 4 – d, 5 – e
C) 1 – b, 2 – c, 3 – d, 4 – e, 5 – a
D) 1 – c, 2 – d, 3 – e, 4 – b, 5 – a
Answer:
A) 1 – c, 2 – e, 3 – d, 4 – a, 5 – b
Question 52.
Choose for correct matching.
Group – A — Group – B
1. Gold — a) thermometers
2. Iron — b) electric wires
3. Aluminium — c) wrapping food
4. Carbon — d) jewellery
5. Copper — e) machinery
6. Mercury — f) fuel
A) 1 – d, 2 – c, 3 – e, 4 – f, 5 – b, 6 – a
B) 1 – d, 2 – e, 3 – c, 4 – f, 5 – b, 6 – a
C) 1 – a, 2 – b, 3 – c, 4 – d, 5 – e, 6 – f
D) 1 – b, 2 – c, 3 – d, 4 – e, 5 – f, 6 – a
Answer:
B) 1 – d, 2 – e, 3 – c, 4 – f, 5 – b, 6 – a
Question 53.
The given apparatus is required to test for sonority.
A) Acid
B) Litmus paper
C) Battery
D) Any apparatus not required
Answer:
D) Any apparatus not required
Question 54.
The simple test for sonority is
A) Heating the metal
B) Dropping the metal on a concrete floor
C) Bending the metcil
D) Dropping the metal in water
Answer:
B) Dropping the metal on a concrete floor
![]()
Question 55.
To test malleability of a substance, the given apparatus is required
A) Hammer
B) Nail
C) Screwdriver
D) Saw
Answer:
A) Hammer
Question 56.
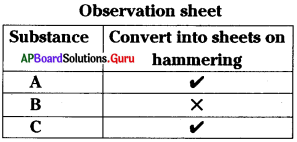
This test is meant for
A) Sonority
B) Malleability
C) Ductility
D) None
Answer:
B) Malleability
Question 57.

The given experiment is used to test the following property of metal
A) Sonority
B) Lustrous
C) Electric conductivity
D) Above all
Answer:
C) Electric conductivity
![]()
Question 58.
What are the materials required to test a metal for electric conductivity?
A) Battery, bulb and connecting wires
B) Hammer, cutter
C) Tester
D) Wax, spirit lamp and pins
Answer:
A) Battery, bulb and connecting wires
Question 59.
The experiment in the given figure is for

A) Conductivity of electricity
B) Condutivity of heat
C) Conductivity of sound
D) Melting of wax
Answer:
B) Condutivity of heat
Question 60.
What precautions do you take while burning sulphur?
A) Do not inhale the flumes
B) Don’t stay against the wind direction
C) Both A and B
D) Neither A nor B
Answer:
C) Both A and B
Question 61.
If you burn sulphur in your lab, it gives
A) Glazing of light
B) It leaves flumes
C) Both A and B
D) Neither A nor B
Answer:
B) It leaves flumes
![]()
Question 62.
Which of the following is used to test for acidity of a substance?
A) Litmus paper
B) Burning test
C) Electrical circuit
D) Any one of the above
Answer:
A) Litmus paper
Question 63.
If you dip blue litmus paper in sulphur dioxide solution, it will change into
A) red colour
B) blue colour
C) yellow colour
D) white colour
Answer:
A) red colour
Question 64.
By the given process you can identify the gas
Process:
1) Bring a burning match stick nearer to the mouth of the test tube with zinc dust and dil HCl.
2) The fire of match stick will put off with pop sound.
A) Oxygen
B) Hydrogen
C) CO2
D) Cl2
Answer:
B) Hydrogen
Question 65.
If you add Zn in copper sulphate (CuSO4) solution, you may observe in the beaker
A) The blue colour of liquid disappears
B) Red colour mass is deposited at the bottom
C) Both ‘A’ and ‘B’
D) Neither A’ nor ‘B’
Answer:
C) Both ‘A’ and ‘B’
![]()
Question 66.
If you drop iron nails in a beaker with copper sulphate, your observations are
A) Red copper is formed on the nails
B) Solution is changed into light green colour
C) Both ‘A’ and ‘B’
D) Neither ‘A’ nor ‘B’
Answer:
C) Both ‘A’ and ‘B’
Question 67.
Early men used these metals to make his tools first
A) iron and copper
B) gold and silver
C) aluminium and gold
D) silver and mercury
Answer:
A) iron and copper
Question 68.
……….. is highly malleable.
A) Gold
B) Iron
C) Sodium
D) Mercury
Answer:
A) Gold
Question 69.
…………. mixture is used in currency coins.
A) Aluminium and copper
B) Iron
C) Gold
D) Silver
Answer:
A) Aluminium and copper
![]()
Question 70.
Maximum metals are obtained in ………… state.
A) Liquid
B) Solid
C) Gaseous
D) Plasma
Answer:
B) Solid
Question 71.
Most ductile metal is
A) Copper
B) Silver
C) Gold
D) Aluminium
Answer:
C) Gold
Question 72.
Which non-metal is dissolved in tincture?
A) chlorine
B) bromine
C) iodine
D) fluorine
Answer:
C) iodine
Question 73.
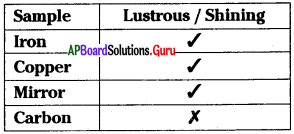
Which of the above materials are non-metals?
A) Iron, copper, mirror
B) Carbon
C) Mirror, carbon
D) None
Answer:
C) Mirror, carbon
![]()
Question 74.

From the table, the correct sentence is
A) All substances are shining substances
B) All shining substances are not metals
C) Some non metals may shine
D) Both B and C
Answer:
D) Both B and C
Question 75.

From the given table which one is not a metal? (mercury is not considered here)
The above substances are beaten hardly by a hammer
A) A
B) B
C) C
D) Both A and C
Answer:
D) Both A and C
Question 76.

The given diagram shows the property of
A) Ductility
B) Malleability
C) Lustrous
D) Sonority
Answer:
A) Ductility
![]()
Question 77.

From this table non-metals are
A) A, B
B) B, C
C) C, D
D) We cannot decide
Answer:
C) C, D
Question 78.
Copper sulphate + Zinc → Zinc sulphate + Copper
Copper sulphate + Iron → Iron sulphate + Copper
Ferrous sulphate + Copper → No reaction
From this experiment more reactive metal is
A) Copper
B) Zinc
C) Iron
D) None
Answer:
A) Copper
Question 79.
Which of the following gives an acid?
A) Sulphur
B) Carbon
C) Magnesium
D) Both A and B
Answer:
D) Both A and B
Question 80.

Adjacent figure gives information about
A) Tools
B) Hammer
C) Early tools
D) All the above
Answer:
C) Early tools
![]()
Question 81.
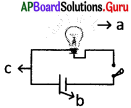
Find the wrong in the given figure
A) a
B) b
C) c
D) None
Answer:
A) a
Question 82.

Identify the wrong in the drawing
A) a
B) b
C) c
D) d
Answer:
C) c
Question 83.
‘X’ is a
A) Conductor of electricity
B) Conductor of head
C) Conductor of sound
D) Any one of the above
Answer:
A) Conductor of electricity
Question 84.
This is appreciable, because majority of metals are obtained from it
A) Air
B) Water
C) Sea
D) Earth
Answer:
D) Earth
![]()
Question 85.
Beautiful jewellery are made with gold. Gold is precious. Because,
A) Gold doesn’t not react with air
B) Gold is not lustruous
C) Gold is not malleable
D) Above all
Answer:
A) Gold doesn’t not react with air
Question 86.
Metals and non-metals are appreciable because, they are widely using in
A) making of acids and bases
B) electrical and household appliances
C) agricultural, constructional tools
D) above all
Answer:
D) above all
Question 87.
The handles of the utensils are made up of
A) metals
B) non-metals
C) both A and B
D) bakelite
Answer:
D) bakelite
Question 88.
………… is found in onions and eggs.
A) sodium
B) silver
C) sulphur
D) none
Answer:
C) sulphur
![]()
Question 89.
The non-metal used in fire works
A) Chlorine
B) Iodine
C) Sulphur
D) Oxygen
Answer:
C) Sulphur
Question 90.
The non-metal used in matchsticks is
A) Chlorine
B) Sulphur
C) Iodine
D) Oxygen
Answer:
B) Sulphur
Question 91.
The non-metal present in garlic, eggs, hair and nails is
A) Chlorine
B) Iodine
C) Oxygen
D) Sulphur
Answer:
D) Sulphur
Question 92.
Metals produce ringing sound when they are beaten by hammer is called
A) malleability
B) ductility
C) sonorous
D) lustrous
Answer:
C) sonorous
![]()
Question 93.
………… foil used in inner packing of food materials and toffees.
A) Sodium
B) Magnesium
C) Aluminium
D) Silicon
Answer:
C) Aluminium
Question 94.
………… mixture is used in currency coins, metals and statues.
A) Aluminium and Copper
B) Aluminium and Zinc
C) Aluminium and Silver
D) Aluminium and Gold
Answer:
A) Aluminium and Copper
Question 95.
Human body contains ………… percent of oxygen.
A) 20
B) 35
C) 75
D) 65
Answer:
D) 65
Question 96.
Which non-metal is present in eggs?
A) sulphur
B) nitrogen
C) chlorine
D) hydrogen
Answer:
A) sulphur
![]()
Question 97.
Which of these metals are useful in preparation of jewellery?
A) Gold
B) Silver
C) Copper
D) All the above
Answer:
D) All the above
Question 98.
The liquid present in thermometer is
A) water
B) alcohol
C) mercury
D) ammonia
Answer:
C) mercury
Question 99.
Choose for correct matching.
Group – A — Group – B
1. Iodine — a) gun powder
2. Silver — b) packing of food material
3. Aluminium — c) medical purpose
4. Oxygen — d) jewellery
5. Sulphur — e) living things need to live
A) 1 – a, 2 – b, 3 – c, 4 – d, 5 – e
B) 1 – b, 2 – c, 3 – d, 4 – e, 5 – a
C) 1 – c, 2 – d, 3 – b, 4 – e, 5 – a
D) 1 – d, 2 – e, 3 – a, 4 – b, 5 – c
Answer:
C) 1 – c, 2 – d, 3 – b, 4 – e, 5 – a
Question 100.
Generally, sonority property of metals is used in the making of
A) Jewellery
B) Horns
C) Bells
D) Conductors
Answer:
C) Bells
![]()
Question 101.
If you want to make wires with a metal, you must select a metal with
A) more sonority
B) more ductility
C) more conductivity
D) more lustrous
Answer:
B) more ductility
Question 102.
A current tester has a plastic / rubber handle instead of metal handle, because
A) rubber / plastic does not conduct heat
B) rubber / plastic does not conduct electricity
C) rubber / plastic is a hard metal
D) plastic / rubber is a soft metal
Answer:
B) rubber / plastic does not conduct electricity
Question 103.
The given property is not important in making of Jewellery
A) Sonority
B) Lustrous
C) Malleability
D) Ductility
Answer:
A) Sonority
Question 104.
Cooking utensils are made with plastic handles because they do not have the property of
A) electrical conductivity
B) heat conductivity
C) lustruous
D) sonority
Answer:
B) heat conductivity
![]()
Question 105.
Srinu: Metals are used in making of cooking vessels due to their property of heat conductivity.
Mohan: Non-metals are used in making of handles of cooking vessels due to their property of heat conductivity.
Which one is correct?
A) Srinu
B) Mohan
C) Both A and B
D) Neither Srinu nor Mohan
Answer:
A) Srinu
Question 106.
Onions contain
A) Carbon
B) Sulphur
C) Ferrous
D) Zinc
Answer:
B) Sulphur
Question 107.
Water purifiers contains activated carbon. It is used as a
A) Purifier
B) Germs killer
C) De colourising agent
D) Sweatner
Answer:
C) De colourising agent
Question 108.
Ajith observed a thin foils over sweets in a sweet stall. This is made up of
A) Silver
B) Gold
C) Iron
D) Copper
Answer:
A) Silver
![]()
Question 109.
Aluminium and copper mixture is used in making of
A) Coins
B) Medals
C) Statues
D) Above all
Answer:
D) Above all
Question 110.
Which gas is evolved when metals react with acids?
A) Oxygen
B) Hydrogen
C) Carbon dioxide
D) Carbon monoxide
Answer:
B) Hydrogen
Question 111.
Match the following.
Group – A — Group – B
1) Sulphur — a) Foil decorated on sweets
2) Silver — b) Making of coins
3) Copper — c) Making fireworks
Choose the correct answer.
A) 1-b, 2-c, 3-a
B) 1-a, 2-c, 3-b
C) 1-c, 2-a, 3-b
D) 1-c, 2-b, 3-a
Answer:
C) 1-c, 2-a, 3-b
Question 112.
Why don’t we use a metallic handle to an electric tester?
A) Metals are good conductors of electricity
B) Metals are highly expensive
C) Metals are rarely occurs
D) Metals are bad conductor of electricity
Answer:
A) Metals are good conductors of electricity
![]()
Question 113.
Which of the following substance is used in preparation of oxygen in the laboratory?
A) Potassium Permanganate
B) Potassium Chloride
C) Ammonium Chloride
D) Copper Sulphate
Answer:
A) Potassium Permanganate
Question 114.
Sulphur dioxide is
A) basicoxide
B) acidicoxide
C) neutraloxide
D) amphotericoxide
Answer:
B) acidicoxide
Question 115.
Match the following.
i. Metal does not have malleability a. Mercury
ii. Metal have malleability b. Phosphorous
iii. Non-metal c. Iron
A) i – c, ii – b, iii – a
B) i – a, ii – b, iii – c
C) i – c, ii – a, iii – b
D) i – a, ii – c, iii – b
Answer:
D) i – a, ii – c, iii – b
![]()
Question 116.
When magnesium is burnt in Oxygen, the formed product dissolves in wa¬ter which is tested with red litmus. Assume the final result in the above experiment.
A) Magnesium oxide is basic in nature
B) Magnesium oxide is neutral in nature
C) Magnesium is a non-metal
D) Magnesium oxide is acidic in nature
Answer:
A) Magnesium oxide is basic in nature
Question 117.
Which of the following is correct question arising in Laxmi’s mind, when she observed vessel on Gas stove with plastic covered handle?
A) Why plastics are used to cover electric wires?
B) Why plastics are used to manufacture water bottles?
C) Why plastics are strong?
D) Why plastics by used for metallic vessel Grips?
Answer:
D) Why plastics by used for metallic vessel Grips?
Question 118.
Assertion (A): Iron can be drawn into wires and used for fencing.
Reason (R): Iron has the property of ductility.
A) Both A and R are correct but R is not correct reason for A
B) A is correct and R is not correct
C) Both A and R are not correct
D) Both A and R are correct and R is correct reason for A
Answer:
D) Both A and R are correct and R is correct reason for A
Question 119.
You know metals conduct heat energj What are precautions to be takei while preparing dosa pan?
A) Pan must be big in size
B) Pan must be small in size
C) Pans must be prepared with heat resistant material
D) Cover the handle of pan with hea resistant material
Answer:
D) Cover the handle of pan with hea resistant material
![]()
Question 120.
Metals used in the preparation or ornaments and resistant to rust
i) Mercury
ii) Gold
iii) Silver
iv) Platinum
A) ii and iv
B) ii and iii
C) ii, iii and iv
D) i only
Answer:
A) ii and iv
Question 121.
Generally metals get rusted. Which of following cases does iron get rusted?
A) In the presence of Oxygen
B) In the presence of moist oxygen
C) In the presence of moist free oxygen
D) In the presence of moisture
Answer:
B) In the presence of moist oxygen
Question 122.
Jaya has taken an iron rod and fixed pins with the help of wax on one side. On the other side, she heated the iron rod. By observing the phenomena that she came to know
a) Wax is melted on heating
b) Iron is a good conductor of heat
c) Iron is an insulator
A) a and b only
B) a and c only
C) a, b and c
D) a only
Answer:
A) a and b only
Question 123.
Silver foils are used to decorate sweets based on the following property.
A) Malleability
B) Sonarity
C) Appearance
D) Ductility
Answer:
A) Malleability
![]()
Question 124.
Which of the following compounds are formed when non – metals react with water?
A) Bases are formed
B) Non-metallic oxides are formed
C) Metallic oxides are formed
D) Acids are formed
Answer:
D) Acids are formed
Question 125.
Identify the correct indicators to observe the properties of metals.
A) Appearance, Ductility
B) Sonarity, Appearance, Ductility, Malleability
C) Chemical properties
D) Sonarity, Appearance
Answer:
C) Chemical properties
Question 126.
i) Copper does not displace Zinc from Zinc Sulphate
ii) Zinc can displace Copper from Copper Sulphate
What do you notice from the above two sentences?
A) High reactive metals can displace less reactive metals from its compound.
B) High reactive metals cannot displace less reactive metals from its compound.
C) Displacement takes place when reactivity of both the metals is equal.
D) Less reactive metals can displace high reactive metals from its compound.
Answer:
A) High reactive metals can displace less reactive metals from its compound.
Question 127.
Observe Set – A, Set – B and choose correct matching.
Set- A — Set-B
a) Sulphur — i) packing covers
b) Carbon — ii) match boxes
c) Aluminium — iii) ornaments
d) Silver — iv) decolourising agent
A) a – ii, b – iv, c – i, d – iii
B) a – iv, b – iii, c – ii, d – i
C) a – iii, b – ii, c – i, d – iv
D) a – i, b – ii, c – iii, d – iv
Answer:
A) a – ii, b – iv, c – i, d – iii
![]()
Question 128.
Non-metals react with Oxygen to give Oxides “X” while metals react with Oxygen to give Oxides “Y”. The chemical nature of “X” and “Y” is
A) X: acidic, Y : basic
B) X : basic, Y : acidic
C) X : acidic, Y : acidic
D) X : basic, Y : basic
Answer:
A) X: acidic, Y : basic
Question 129.
Match the three major elements found in the human body with the percentages.
Element Percentage
1. Hydrogen a) 65%
2. Oxygen b) 18%
3. Carbon c) 10%
d) 0.04%
A) 1-d, 2-b, 3-a
B) 1-c, 2-a, 3-b
C) 1-a, 2-b, 3-c
D) 1-b, 2-c, 3-d
Answer:
B) 1-c, 2-a, 3-b
Question 130.
Most metals react with dilute acids to liberate Hydrogen gas. Which metal among the following does not?
A) Magnesium
B) Aluminium
C) Iron
D) Copper
Answer:
D) Copper
Question 131.
The non – metal which is in solid state at room temperature
A) Carbon
B) Chlorine
C) Bromine
D) Iodine
Answer:
A) Carbon
![]()
Question 132.
The main apparatus required in the activity to observe the heat conductivity of metals
A) Metal pins
B) Retard stand
C) Spirit lamp
D) All the above
Answer:
D) All the above
Question 133.
The metal that is present in haemoglobin
A) Magnesium
B) Iron
C) Carbon
D) Zinc
Answer:
B) Iron
Question 134.
Metals are generally solid. Which of the following metals is in the liquid state at room temperature?
A) Mercury
B) Silver
C) Aluminium
D) Sodium
Answer:
A) Mercury
Question 135.
Magnesium ribbon on burning in air produces
A) Magnesium oxide, heat and light
B) Magnesium oxide, heat
C) Magnesium oxide, water and light
D) Magnesium oxide, heat and light
Answer:
A) Magnesium oxide, heat and light
![]()
Question 136.
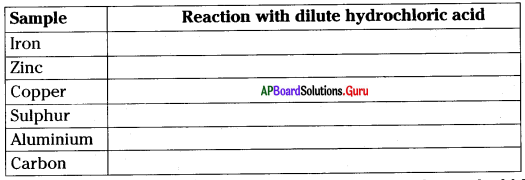
Take the samples given in the table in different test tubes and add 5 ml of dilute HCl (hydrochloric acid) to it.
If you see a reaction in any test tube, bring a burning matchstick near the mouth of the test tube and see if you hear a pop sound. Which of the following can be concluded based on this experiment?
A) Some non – metals react with HCl releasing chlorine gas.
B) Some metals react with HCl releasing hydrogen gas.
C) Some non-metals react with HCl releasing hydrogen gas.
D) Some metals and non-metals undergo rusting on reacting with HCl.
Answer:
B) Some metals react with HCl releasing hydrogen gas.
Question 137.
Which of the following is used to check the acidic or basic nature of a substance?
A) Magnet
B) Burning matchstick
C) Kerosene
D) Litmus paper
Answer:
D) Litmus paper
The human body is made up of the following elements: Oxygen (65%), Carbon (18%), Hydrogen (10%), Nitrogen (3%), Calcium (1.5%), Phosphorus (1%).
Based on above information answer following two questions:
Question 138.
The highest percentage of element present in our body is
A) Carbon
B) Oxygen
C) Calcium
D) Hydrogen
Answer:
B) Oxygen
![]()
Question 139.
How many elements are present in our body?
A) 5
B) 6
C) 7
D) 8
Answer:
B) 6