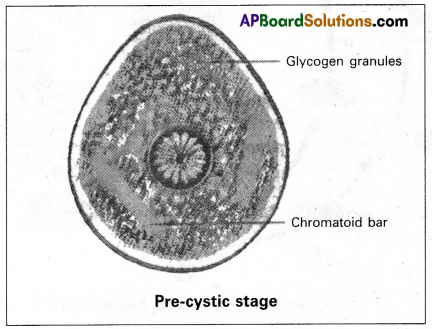
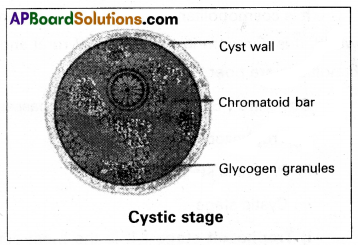
Andhra Pradesh BIEAP AP Inter 2nd Year Zoology Study Material Lesson 1(a) Digestion and Absorption Textbook Questions and Answers.
AP Inter 2nd Year Zoology Study Material Lesson 1(a) Digestion and Absorption
Very Short Answer Questions
Question 1.
Give the dental formula of an adult human being.
Answer:
The arrangement of different types of teeth in each half of both the jaws in the order I, C, PM, M is represented by the dental formula.
In adult it is = [latex]\frac{2123}{2123}[/latex] = 32.
Question 2.
Bile juice contains no digestive enzymes, yet it is important for digestion. How?
Answer:
Bile juice doesn’t contain enzymes, but it contains bile salts such as sodium/potassium glycocholates and taurocholates, which help in the digestion and absorption of lipids. Bile salts emulsify fats and also render them water-soluble. Bile salts activate the lipases. These lipases act on emulsified fats and convert them into fatty acids and glycerols.
Question 3.
Describe the role of chymotrypsin. Name two other digestive enzymes of the same category secreted by the same gland.
Answer:
Chymotrypsin plays an important role in digestion of proteins, proteoses and peptones and convert them into tripeptides and dipeptides. Chymotrypsin, trypsin and carboxy peptidase are ehdopeptidases, produced by the pancreas arid involved in the digestion of proteins.
Question 4.
What would happen if, HCl were not secreted in the stomach?
Answer:
HCl is secreted by the glands present on the stomach walls. It provides acidic pH which is optimal for the action of pepsin. HCl activates the pepsinogen into pepsin. Pepsin plays an important role in digestion of proteins. Therefore, if HCl were not secreted in the stomach, then pepsin would not be activated. This would affect on protein digestion.
Question 5.
Explain the terms thecodont and diphyodont dentitions.
Answer:
Thecodont:
Teeth of human beings are embedded in the sockets of the jaw bones is called thecodont.
Diphyodont:
Majority of mammals including human beings form two sets of teeth during their life time, a set of temporary / milk teeth replaced by a set of permanent teeth. This type of dentition is called diphyodont dentition.
![]()
Question 6.
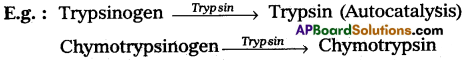
What is Autocatalysis? Give two examples.
Answer:
Autocatalysis is the catalysis of a reaction in which the catalyst is one of the product of the reaction (or) catalysis caused by a catalytic agent formed during a chemical reaction.
Question 7.
What is chyme?
Answer:
Semi fluid mass of partly digested acidic food formed in the stomach is called chyme.
Question 8.
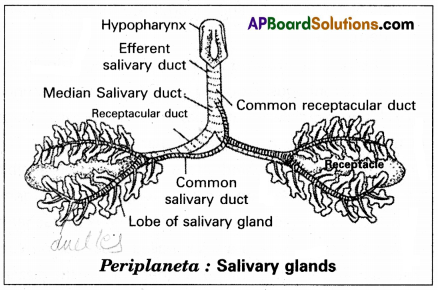
Name the different types of Salivary glands of man, and their locations in the human body?
Answer:
There are three pairs of Salivary glands in man.
1. Parotid glands — present below the pinna / inner surface of the cheeks.
2. Sub maxillary (or) Sub mandibular glands —.located at the angles of lower jaw.
3. Sublingual glands — present below the tongue.
Question 9.
Name different types of papillae present on the tongue of man.
Answer:
The upper surface of the tongue has small projections called papillae. In humans the tongue bears 3 (three) types of papillae namely 1) fungi form 2) filiform 3) Circumvallate papillae.
Question 10.
What is the hardest substance in the human body? What is its origin?
Answer:
Enamel of tooth is the hardest substance in the human body, which is secreted by ameloblasts of ectodermal origin.
Question 11.
Name the structure of gut which is vestigial in human beings, but well developed in herbivores. And mention the type of tissue with which it is mostly formed.
Answer:
Appendix is vestigial part in human beings. It is a narrow finger like tubular projection, arises from the caecum. In herbivores it is a functional part and useful in the digestion of cellulose materials. The appendix contain a high concentration of lymphoid tissues. These are highly specialized structures which are a part of the immune system.
Question 12.
Distinguish between deglutition and mastication.
Answer:
Deglutition :
Deglutition is the swallowing of food and involves a complex and coordinated process. It is divided into three phases.
Phase one :
The collection and swallowing of masticated food.
Phase two :
Passage of food through the pharynx into the beginning of the esophagus.
Phase three :
The passage of food into the stomach.
Mastication :
The mastication process includes the biting and tearing of food into manageable pieces. This usually involves using the incisors and canines teeth. The grinding of food is usually performed by the molars and premolars. During the mastication process, food is moistened and mixed with saliva.
![]()
Question 13.
Distinguish between diarrhoea and constipation.
Answer:
Diarrhoea :
The abnormal frequency of bowel movement and increased liquidity of the faecal discharge is known as diarrhoea. It reduces the absorption of fqod arid results in loss of water.
Constipation :
A condition ip which the faeces are retained within the rectum as it is hard due to low content of water and the movement of bowel occurs irregularly.
Question 14.
Name two hormones secreted by the duodenal mucosa.
Answer:
The epithelium of duodenum secretes the hormones namely secretin and cholecystokinin (cck).
Question 15.
Distinguish between absorption and assimilation.
Answer:
Absorption :
Absorption is the process by which the end products of digestion pass through the intestinal mucosa into blood (or) lymph. It is carried out by passive, active (or) facilitated transport mechanisms.
Assimilation:
The absorbed substances finally reach the tissues, where food materials become integral components of the living protoplasm and used for the production of energy, growth and repair. This process is called assimilation.
Short Answer Questions
Question 1.
Draw a neat labelled diagram of L.S of a tooth. Ans.
Answer:
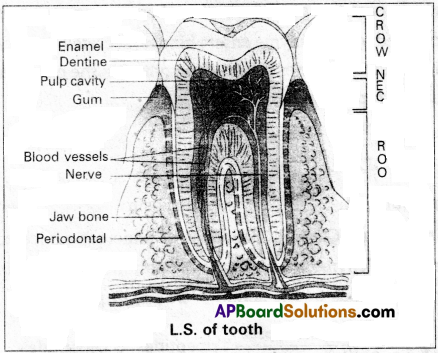
Question 2.
Describe the process of digestion of proteins in the stomach.
Answer:
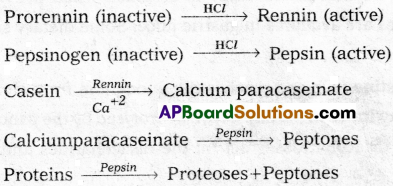
Protein digestion begins in the stomach. The food entered into the stomach is mixed thoroughly with the gastric juice of the stomach by the churning movements of its muscular wall and the product is called chyme. The main components of gastric juice are protein digestive enzymes, hydrochloric acid and mucus.
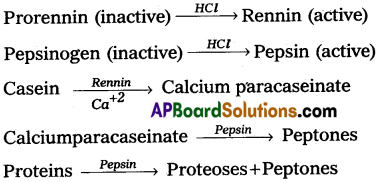
HCl provides the acidic pH (1.8) which is optimal for the action of pepsin. The proenzymes of gastric juice, the pepsinogen and prorennin, on exposure to hydrochloric acid are convened into the active enzymes, pepsin and rennin respectively. Pepsin converts proteins into proteoses and peptones. Rennin is a proteolytic enzyme found in the gastric juice of infants. It acts on the milk protein, the casein in the presence of calcium ions and converts it into calcium paracaseinate and proteoses. Pepsin acts on calcium paracaseinate and converts it into peptones. The entire process of protein digestion in the stomach takes about 4 hours.
Question 3.
Explain the role ofpancreatic juice in the digestion of proteins.
Answer:
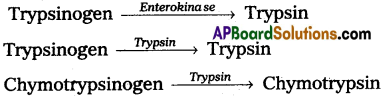
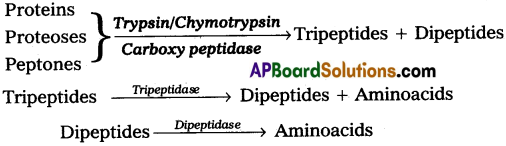
Pancreatic juice is secreted by the pancreas and it plays an important role in protein ‘digestion. Pancreatic juice contains protein hydrolysing enzymes like trypsinogen, chymotrypsinogen and pro carboxy peptidases, but they are inactive enzymes.
Trypsinogen is activated by the enzyme enterokinase, secreted by the intestinal mucosa into active trypsin, which in turn activates the other enzymes in the pancreatic juice. Trypsin itself can similarly activate trypsinogen into trypsin.
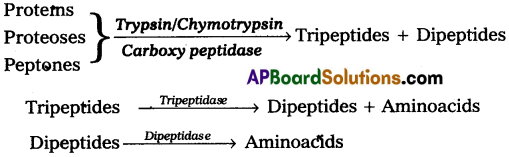
Chymotrypsin, Trypsin and Carboxy Peptidase of pancreatic juice act upon proteins, proteoses and peptones in the chyme, result in the formation of tri and dipeptides. Which in turn hydrolysed into aminoacids by the action Of tri aruj di peptidases.
Question 4.
How are polysaccharides and disaccharides digested?
Answer:
Dietary carbohydrates principally consist of polysaccharides :
Starch and glycogen. It contains disaccharides and small amounts of monosaccharides.
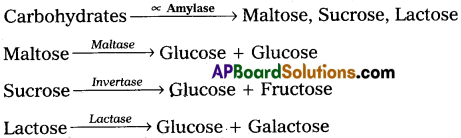
Digestion in mouth:
Digestion of carbohydrates starts at the mouth, where they come in contact with saliva during mastication. Saliva contains carbohydrate-splitting enzyme called Salivay amylase (ptyalin). This enzyme hydrolyses the starch into disaccharides (maltose).
![]()
Digestion in stomach:
Ptyalin action stops in stomach when pH falls to 3.0. No carbohydrate splitting enzymes are available in gastric juice. Some dietary sucrose may be hydrolysed by HCl.
Digestion in small intestine :
Chyme reaches the duodenum from stomach where it meets pancreatic juice. Carbohydrates in the chyme are hydrolysed by the pancreatic amylase into disaccharides. Intestinal disaccharidases act on the disaccharides and convert them into monosaccharides.
![]()
Question 5.
If you take butter in your food how does it get digested and absorbed in the body? Explain.
Answer:
Butter contains fat. Fats remain mostly undigested in stomach.
Digestion of fat in the small intestine :
The major site of fat digestion is the small intestine. This is due to the presence of a powerful lipase/(steapsin) in the pancreatic juice and bile juice. Bile juice contains bile salts such as Sodium/Potassium glycocholates and taurocholates, which helps in the emulsification of fat i.e., break down of fats into very small micelles. Bile also activates lipases of pancreatic juice (steapsin) and intestinal lipases. These lipases act on emulsified fats and convert them into fatty acids and glycerols.
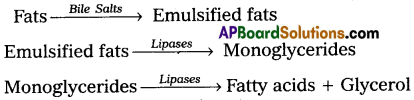
Absorption :
Fatty acids and glycerol being insoluble in water cannot be absorbed into the blood directly. They are first modified into small droplets called micelles, which move into intestinal mucosal cells. They are reformed into very small protein coated fat globules called chylomicrons, which are transported into the lymph capillaries in the villi by exocytosis. Then they are ultimately released into blood stream through left subclavian vein via the thoracic duct. These chylomicrons are broken down to fatty acids and glycerol by the action of an enzyme lipoprotein lipase and they diffuse into the adipocytes of the adipose tissue and liver for storage.
Question 6.
What are the functions of liver?
Answer:
Liver performs a variety of functions such as synthesis, storage and secretion of various substances.
- Liver secretes bile juice, it contains bile salts such as sodium / potassium glycocholates and taurocholates, which helps in digestion and absorption of lipids.
- Liver plays the key role in carbohydrate metabolism.
a) Glycogenesis : formation of glycogen from glucose.
b) Glycogenolysis : breakdown of glycogen into glucose.
c) Gluconeogenesis : Synthesis of glucose from certain amino acids, lactate (or) glycerol. - Liver also plays an important role in synthesis of cholesterol and production of triglycerides.
- Deamination of proteins occurs in the liver.
- Liver is the chief organ of detoxification of toxic substances that enter the gut along with food.
- Liver acts as thermoregulatory organ.
- Liver acts as a haemopoietic organ in the foetus and erythroclastic organ in the adult.
- The liver synthesizes the plasma proteins such as albumin, globulins, blood clotting factors such as fibrinogen / prothrombin, etc., and the anticoagulant called heparin.
- The lactic acid formed during anaerobic muscle contraction is converted into glycogen (gluconeogenesis) in the liver by Cori cycle.
- Kupffer cells are the largest phagocytic cells which remove unwanted substances and microbes that attack the liver by phagocytosis.
Long Answer Questions
Question 1.
Describe the physiology of digestion of various types of food in the human digestive system
Answer:
Digestion is the process of convertion of complex non-diffusible food substances into simple diffusible forms. The process of digestion is accomplished by mechanical and biochemical process.
I. Digestion in the buccal cavity :
Buccal cavity performs two major functions, mastication of food and facilitation of swallowing. Teeth and tongue with the help of saliva masticate and mix up the food thoroughly. Mucus in saliva helps in lubricating and adhering the masticated food particles into bolus. The saliva secreted into oral cavity contains electrolytes such as Na+, K+, Cl–, HCO3– arid enzymes like salivary amylase (ptyalin) and lysozyme. Carbohydrates digestion starts in the buccal cavity, about 30% of starch is hydrolysed here into a disaccharide called maltose by the enzyme amylase (ptyaline). Lysozyme acts as antibacterial agent that prevents infections.
II. Digestion in the stomach :
As the bolus enters into stomach starch digestion stops and protein digestion begins. The food entered into stomach is mixed thoroughly with gastric juice of the stomach by the churning movement of its muscular wall and the product is called chyme. The mucus and bicarbonates present in the gastric juice act as lubricant and protect the mucosal epithelium from HCl. HCl in the stomach provides the acidic pH (1.8) which is optimal for the action of pepsin.
The proenzymes of gastric juice, the pepsinogen and prorennin on exposure to HCl are converted into the active enzymes, pepsin and rennin respectively. Pepsin converts proteins into proteoses and peptones. Rennin found in gastric juice of infants. It acts on the milk protein, the casein in the presence of calcium ions convert into calcium paracaseinate and proteoses. Pepsin acts on paracaseinate and convert it into peptones. The entire process of protein digestion in stomach takes about 4 hours.
III. Digestion in the small intestine :
Various types of movements are generated by the muscular external layer of small intestine. These movements help in thorough mixing of the food with bile, pancreatic juice and intestinal juice in the intestine and thereby facilitate digestion. The duodenal cells of the proximal part produces large amount of bicarbonates to completely neutralize any gastric acid that passes further down into the digestive tract.
i) Digestion of proteins :
Pancreatic juice contains protein hydrolysing enzymes like trypsinogen, chymotrypsin and procarboxy peptidases, but they are inactive enzymes.
Trypsinogen is activated by the enzyme enterokinase secreted by the intestinal mucosa into active trypsin which intum activate the other enzymes in the pancreatic juice. Trypsin itself can similarly activate trypsinogen into trypsin.
Chymotrypsin, trypsin and carboxy peptidase of pancreatic juice act upon proteins, proteoses and peptones in the chyme, result in the formation of tri and dipeptides which inturn hydrolysed into amino acids by the action of tri and dipeptidases.
ii) Digestion of fats:
Bile salts of bile help in the emulsification of fat i.e., breakdown of fats into very small micelles. Bile also activates lipases of pancreatic juice (steapsin) and intestinal lipases. These lipases act on emulsified fats and convert them into fatty acids and glycerols.
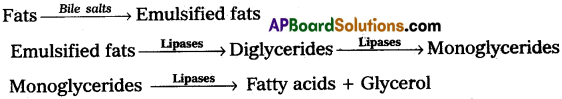
iii) Digestion of Carbohydrates :
Carbohydrates in the chyme are hydrolysed by the pancreatic amylase into disaccharides. Intestinal disaccharidases act on the disaccharides and convert them into monosacharides.

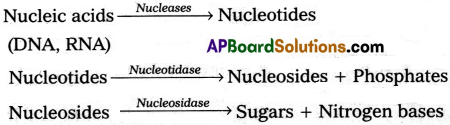
iv) Digestion of nucleic acids :
Nucleases of the pancreatic juice act on the nucleic acids to form nucleotides and nucleosides. Nucleotidases and nucleosidases of the intestinal juice convert the nucleotides and nucleosides into pentose sugar and nitrogen bases.
The end products of digestion pass through the intestinal mucosa into blood (or) lymph is carriedout by passive, active (or) facilitated transport mechanisms.
![]()
Question 2.
Explain the digestive system of man with neat labelled diagram.
Answer:
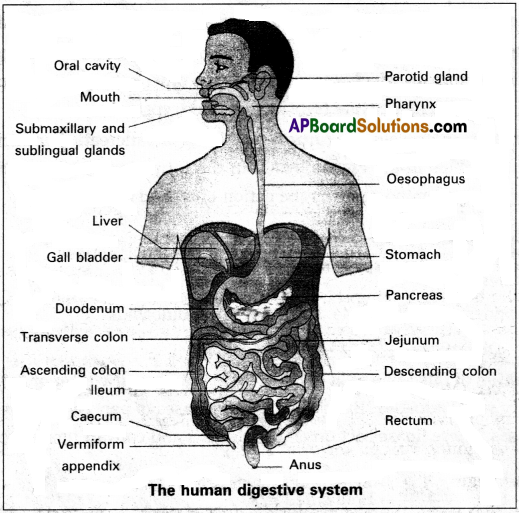
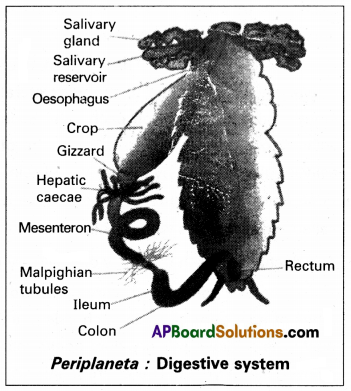
The digestive system is a group of organs and tissues involve in the breaking down of ingested food in the alimentary canal into a form that can be absorbed ai a assimilated by the tissues of the body.
Human digestive system consists of the alimentary canal and the associated glands. Alimentary canal / Digestive tract:
The alimentary canal of man begins with the anterior opening, the mouth and ends with the posterior opening, the anus.
Parts of the alimentary canal / digestive tract:
- Mouth and Buccal (oral) cavity
- Pharynx
- Oesophagus
- Stomach
- Small intestine
- Large intestine
1. Mouth and Buccal (oral) cavity:
Mouth is the first part of the alimentary canal. It is formed by the cheek on either side and boardered by the movable upper and lower lips, leads into the buccal (or) oral cavity. The palate separate the ventral buccal cavity from the dorsal nasal chamber and facilitates chewing and breathing simultaneously. The jaw bones bear teeth and tongue occurs at the base of the buccal cavity.
i) Teeth :
These are ecto-mesodermal in origin. An adult human has 32 permanent teeth, which are of four different types namely, incisors (I), canines (C), premolars (PM), and molars (M). These are useful in cutting, tearing and grinding of food. The arrangement’ of teeth is represented by dental formula. In adult human
it is = [latex]\frac{2123}{2123}[/latex] = 32
ii) Tongue :
It is a freeely movable muscular sense organ, attached to the floor of the oral cavity by a fold of tissue called frenulum. The upper surface of the tongue has small projections called papillae, some of which bear taste buds. The tongue acts as universal toothbrush and helps in mixing saliva with food, taste detection, deglutition and speaking.
2. Pharynx:
It is a muscular tube connecting the oral cavity and oesophagus and trachea. It is a common passage for food and air. It is divided into nasopharynx, oropharynx and laryngo pharynx. Oesophagus and trachea open into the laryngopharynx. The trachea open into the laryngopharynx through the glottis. A cartilaginous flap called epiglottis prevents the entry of food into glottis during swallowing.
3. Oesophagus:
It is a thin long muscular tube (9 to 12 inches). The semisolid digested food from pharynx enters the oesophagus. Oesophagus is separated by the Cardiac sphincter from stomach. When the food reaches lower end of Oesophagus the cardiac sphincter opens allowing the food to enter the stomach.
4. Stomach :
It is a wide ‘J’ shaped muscular sac, located iii the upper left portion of the abdominal cavity just below the diaphragm. It has three major parts, an anterior cardiac portion into which the oesophagus opens, a middle large fundic region and a posterior pyloric portion which opens into the first part of the small intestine through the pyloric aperture which is guarded by the pyloric sphincter.
5. Small intestine :
The small intestine is the longest part of alimentary canal. It has three regions namely proximal duodenum middle long coiled jejunum and distal highly coiled ileum. Duodenum receives the hepato-pancreatic duct. Ileum opens into the large intestine.
6. Large intestine :
It consist of caecum, colon and rectum. Caecum is a small blind sac. A narrow finger like vestigial tubular organ arises from caecum called appendix. The caecum opens into colon which is‘divided into an ascending, a transverse, a descending parts and a sigmoid colon that continues behind into rectum. Rectum is a small dilated sac which leads into anal canal that opens out through the anus.
Digestive glands :
1. Salivary glands : There are three pairs of glands in man.
i) Parotid glands
ii) Sub-maxillary glands
iii) Sub-lingual glands
They secrete saliva, which mainly contains salivary amylase and lysozyme.
2. Gastric glands :
These are located in the wall of the stomach beneath the surface
epithelium, Gastric glands are of three types namely
i) Cardiac glands – secrete mucus
ii) Pyloric glands – secrete mucus and hormone gastrin
iii) Fundic / Oxyntic glands – secrete mucus, proenzymes like pepsinogen and prorennin, HCl, intrensic factor and some amount of gastric lipase.
3. Intestinal glands :
They are of two types
i) Brunner’s glands
ii) Crypts of lieberkuhn
which secrete intestinal juice contains peptidases, disaccharidases, enterokinase and lysozyme.
4. Liver :
Liver is the largest gland in human. Liver secretes bile juice, contains bile salts, which play a very important role in lipid digestion.
5. Pancreas :
The pancreas is the second largest gland in human. Exocrine part of pancreas secretes pancreas juice contains sodium bicarbonates, trypsinogen, chymotrypsinogen, carboxy peptidase, steapsin, -pancreatic amylase and nucleases such as DNAase and RNAase.