AP State Board Syllabus AP SSC 10th Class Chemistry Solutions Chapter 4 Acids, Bases and Salts Textbook Questions and Answers.
AP State Syllabus SSC 10th Class Chemistry Solutions 4th Lesson Acids, Bases and Salts
10th Class Chemistry 4th Lesson Acids, Bases and Salts Textbook Questions and Answers
Review of Your Previous Knowledge
Question 1.
Which property do you think of while suggesting the remedy from a problem of acidity?
Answer:
Neutralization Property. Antacid tablets neutralise acidity.
Improve your learning
Question 1.
Five solutions A, B, C, D and E when tested with universal indicator showed pH as 4, 1, 11,7 and 9 respectively, which solution is : (AS1)
a) neutral
b) strongly alkaline
c) strongly acidic
d) weakly acidic
e) weakly alkaline
Arrange the pH in increasing order of hydrogen ion concentration.
Answer:
Solution – pH Value
A → 4
B → 1
C → 11
D → 7
E → 9
a) Solution ‘D’ is neutral
b) Solution ‘C’ is strongly alkaline
c) Solution ‘B’ is strongly acidic
d) Solution ‘A’ is weakly acidic
e) Solution ‘E1 is weakly alkaline
∴ Increasing order of Hydrogen ion concentration : C < E < D < A < B.
Question 2.
What is a neutralization reaction? Give two examples. (AS1)
Answer:
Neutralization reaction : When acid reacts with base, forms its salt and water. This reaction is called a neutralization reaction.
Examples :

Equation: HCl + NaOH → NaCl + H2O
ii) Acetic Acid + Sodium Hydroxide → Sodium Acetate + Water
Equation : CH3COOH + NaOH → CH3COONa + H2O
Formula : Acid + Base > Salt + Water
Question 3.
What happens when an acid or base is mixed with water? (AS1)
Answer:
When an acid or base is mixed with water it changes into dilute acid or dilute base.
(OR)
Dilute acid or dilute base will be formed when an acid or base is mixed with water. Mixing an acid or base with water results in decrease in the concentration of ions (H30+/ OH-) per unit volume. Such a process is called dilution and the acid or base is said to be diluted.
![]()
Question 4.
Why does tooth decay start when the pH of mouth is lower than 5.5? (AS1)
(OR)
Does the pH change tooth decay? Explain.
Answer:
- Tooth enamel is the hardest substance in the body.
- It doesn’t dissolve in water but corroded when the pH in the mouth is below 5.5.
- It happens due to the bacteria which produce acids by degradation of sugar and food particles remaining in the mouth.
Question 5.
Why does not distilled water conduct electricit? (AS2)
Answer:
- Distilled water does not contain impurities.
- It is also extremely weak electrolyte.
- So it does not dissociate into ions.
- It does not have charge carriers.
- Because of that it does not conduct electricity.
Question 6.
Dry hydrogen chloride gas does not turn blue litmus to red whereas hydrochloric acid does. Why? (AS1)
Answer:
1. Dry hydrogen chloride gas is not an acid. Because it does not produce H+(aq) ions. Hence it can’t turn blue litmus into red.
2. Hydrochloric acid is an aqueous solution. So it can produce H+(aq) ions. Hence it can turn blue litmus into red.
Question 7.
Why pure acetic acid does not conduct electricity? (AS1)
Answer:
The reasons for pure acetic acid does not conduct electricity are :
i) Acetic acid is a weak acid.
ii) It gives fewer H3O+ ions.
![]()
Question 8.
A milkman adds a very small amount of baking soda to fresh milk. (AS2)
a) Why does he shift the pH of the fresh milk from 6 to slightly alkaline?
Answer:
1. By adding a very small amount of baking soda to fresh milk, the milkman keeps the milk unspoiled for little more time than usual time.
2. As the pH value increases the milk turns to slightly alkaline.
b) Why does this milk take a long time to set as curd?
Answer:
- Curd form from the milk by the action of Lactic acid produced by bacteria in the milk.
- If milk man add Baking soda (NaHCO3) to the milk it neutralise acid, which is produced by the bacteria.
- Excess acid is required to change the milk as curd.
- It takes long time.
Question 9.
Plaster of Paris should be stored in a moisture-proof container. Explain why? (AS2)
Answer:
Storing of Plaster of Paris :
- Plaster of Paris is a white powder.
- It easily absorbs water in air and forms hard gypsum.
- So, it should be stored in a moisture-proof container.
Question 10.
Fresh milk has a pH of 6. Explain why the pH changes as it turns into curd.
Answer:
1. Fresh milk has a pH of 6. Hence it is a weak acid.
2. To turn the milk as curd, we have to add yeast in the form of some curd. The fermentation takes place during this process and lactose changes in lactic acid and the pH decreases as milk sets as curd.
![]()
Question 11.
Compounds such as alcohols and glucose contain hydrogen but are not categorized as acids. Describe an activity to prove it. (AS3)
(OR) (Activity – 7)
Write an activity to show that the solutions of compounds like alcohol and glucose do not show acidic character even though they are having Hydrogen.
(OR)
Write an activity which proves acids are good conductors of electricity.
(OR)
The acidity of acids is attributed to the H+ ions produced by them in solution explain the above statement with an activity.
List out the material for the experiment to investigate whether all compounds containing Hydrogen are acids or not and write the experimental procedure.
Answer:
List of the material required :
- Glucose
- Alcohol
- Dil. HCl
- Dil-H2SO4
- Beaker
- Connecting wires
- 230 voltage AC supply
- Bulb
- Graphite rods.
Procedure :
- Prepare glucose, alcohol, hydrochloric acid and sulphuric acid solutions.
- Connect two different coloured electrical wires to graphite rods separately as shown in figure.
- Connect free ends of the wire to 230 volts AC plug.
- Complete the circuit as shown in the figure by connecting a bulb to one of the wires.
- Now pour some dilute HCl in the beaker and switch on the current.
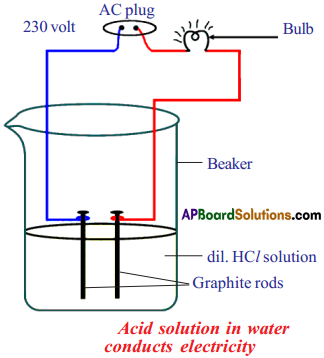
Observation :
The bulb starts glowing.
Repetition:
Repeat activity with dilute sulphuric acid, glucose and alcohol solutions separately.
Observation :
- We will notice that the bulb glows only in acid solutions.
- But the bulb does not glow in glucose and alcohol solutions.
Result:
- Glowing of bulb indicates that there is flow of electric current through the solution.
- Acid solutions have ions and the movement of these ions in solution helps for flow of electric current through the solution.
Conclusion :
- The positive ion (cation) present in HCl solution is H+.
- This suggests that acids produce hydrogen ions H+ in solution, which are, responsible for their acidic properties.
- In glucose and alcohol solution the bulb did not glow indicating the absence of H+ ions in these solutions.
- The acidity of acids is attributed to the H+ ions produced by them in solutions.
Question 12.
What is meant by “water of crystallization” of a substance? Describe an activity to show the water of crystallisation. (Activity – 16) (AS3)
Answer:
Water of Crystallization : Water of crystallization is the fixed number of water molecules present in one formula unit of a salt in its crystaline form.
Ex : CuSO4 • 5H2O.
It means that five water molecules are present in one formula unit of copper sulphate.
Activity to show the water of crystallization :
- Take a few crystals of copper sulphate in a dry test tube and heat the test tube.
- We observe water droplets on the walls of the test tube and salt turns white.
- Add 2 – 3 drops of water on the sample of copper sulphate obtained after heating.
- We observe, the blue colour of copper sulphate crystals is restored.
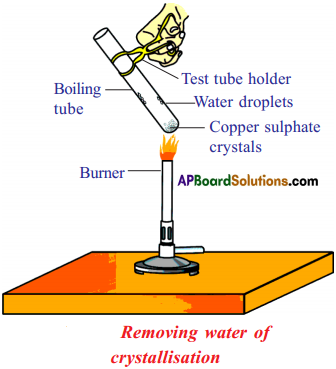
Reason :
1. In the above activity copper sulphate crystals which seem to be dry contain the water of crystallization, when these crystals are heated, water present in crystals is evaporated and the salt turns white.
2. When the crystals are moistened with water, the blue colour reappears.
Removing water of crystallization
![]()
Question 13.
Equal lengths of magnesium ribbons are taken in test tubes A and B. Hydrochloric acid is added to test tube A, while acetic acid is added to test tube B. Amount and concentration of both the acids are same. In which test tube will the fizzing occur more vigorously and why? (AS4)
Answer:
1. The volatility of acetic acid (CH3COOH) is more than that of hydrochloric acid.
2. But HCl solution has more strength than acetic acid.
3. Hence magnesium ribbon in test tube A will react more vigorously than in B.
4. So fizzing occurs more vigorously in test tube ‘A’.
Question 14.
Draw a neat diagram showing acid solution in water conducts electricity. (AS5)
(OR)
Draw a neat diagram which shows acids contains H+ ions.
(OR)
Draw a neat diagram showing how does dilute HCl solution conduct electricity.
Answer:

Question 15.
How do you prepare your own indicator using beetroot ? Explain. (AS5)
Aim : To prepare own indicator.
Materials required :
1) Beetroots-2 or 3
2) Knife
3) Bowls
4) Water
5) Spoon
6) Mixy
7) Orange juice
Procedure:
1) Take the beetroots and peel them with the help of a knife. (Firstly wash them).
2) Chop them into pieces.
3) Put those pieces into a mixy jar and make a paste.
4) Add some water to the paste. Now filter this and collect only juice from this.
Observation and Result:
1) Now add 5 to 6 drops of this juice, (beetroot juice (indicator)) to orange juice (5 to 6 drops) and mix it.
2) We can see the colour changes. This indicates the presence of acidic nature in orange juice.
Question 16.
How does the flow of acid rain into a river make the survival of aquatic life in a river difficult? (AS7)
(OR)
What are the harmful effects of acid rain?
Answer:
1) Acid rains are combination of carbonic acid, sulphuric acid and nitric acid with rain water.
2) The pH of acid rain is less than 5.6.
3) Living organisms can survive only in a narrow range of pH change.
4) When acid rain with pH value less than 5.6, flows into rivers, it lowers the pH of river water.
5) Due to less pH, the river water becomes acidic and hence the aquatic life in such rivers becomes difficult.
Question 17.
What is baking powder? How does it make the cake soft and spongy? (AS7)
Answer:
1) Baking Powder:
Baking powder is a mixture of baking soda (NaHCO3) and a mild edible acid such as tartaric acid. COOH (CHOH)2 COOH
2) Chemical reaction :
When baking powder is heated or mixed in water, the following reaction takes place.
NaHCO3 + H+ → CO2 + H2O + Sodium salt of acid.
3) Carbondioxide produced during the reaction causes bread or cake to rise making them soft and spongy.
![]()
Question 18.
Give two important uses of washing soda and baking soda. (AS7)
(OR)
Write the chemical formulae for washing soda and Baking soda and give their uses.
(OR)
Write any four uses of washing soda.
Answer:
Uses of washing soda (Na2CO3.10H2O) :
1) Washing soda (sodium carbonate) is used in glass, soap and paper industries.
2) It is used in the manufacture of sodium compounds such as borax.
3) Sodium carbonate can be used as a cleaning agent for domestic purposes.
4) It is used for removing permanent hardness of water.
Uses of baking soda (NaHCO3 10H2O) :
1) Baking soda (Sodium hydrogen carbonate) is used for faster cooking.
2) Baking powder (a mixture of baking soda and a mild acid) is used in preparation of cakes.
3) Sodium hydrogen carbonate is also an ingredient in antacids.
4) It is also used in soda – acid, fire extinguishers.
5) It acts as a mild antiseptic.
Fill in the Blanks
1. i) ………………….. taste is a characteristic property of all acids in aqueous solution.
ii) Acids react with some metals to produce ………………….. gas.
iii) Because aqueous acid solutions conduct electricity, they are identified as …………………..
iv) Acids react with bases to produce a ………………….. and water.
v) Acids turn methyle orange into ………………….. colour.
Answer:
1. i) Sour
ii) hydrogen
iii) electrolytes or conductors
iv) salt
v) red
![]()
2. i) Bases tend to taste ………………….. and feel ………………….. .
ii) Like acids, aqueous basic solutions conduct ………………….., and are identified as ………………….. .
iii) Bases react with ………………….. to produce a salt and
iv) Bases turn phenophthalein into ………………….. colour.
Answer:
2. i) bitter, soapy (slippery) to touch
ii) electricity, electrolytes
iii) acids, water
iv) pink
Match the following :
| a) Plaster of Paris | 1) CaOCl2 |
| b) Gypsum | 2) NaHCO3 |
| c) Bleaching powder | 3) Na2CO3 |
| d) Baking soda | 4) CaSO4.½H2O |
| e) Washing soda | 5) CaSO4.2H2O |
Answer:
3. a – 4,
b – 5,
c – 1,
d – 2,
e – 3.
Multiple Choice Questions
1. The colour of methyl orange indicator in acidic medium is
A) yellow
B) green
C) orange
D) red
Answer:
D) red
2. The colour of phenolphthalein indicator in basic solution is
A) yellow
B) green
C) pink
D) orange
Answer:
C) pink
![]()
3. Colour of methyl orange in alkali conditions
A) orange
B) yellow
C) red
D) blue
Answer:
B) yellow
4. A solution turns red litmus blue, its pH is likely to be
A) 1
B) 4
C) 5
D) 10
(OR)
If a solution converts red litmus into blue colour, then its pH value is …………….. .
A) 1
B) 4
C) 5
D) 10
Answer:
D) 10
![]()
5. A solution reacts with crushed egg-shells to give a gas that turns lime-water milky, the solution contains …………….. .
A) NaCl
B) HCl
C) LiCl
D) KCl
Answer:
B) HCl
6. If a base dissolves in water, by what name is it better known?
A) neutralization
B) basic
C) acid
D) alkali
Answer:
D) alkali
7. Which of the following substances when mixed together will produce table salt?
A) Sodium thiosulphate and sulphur dioxide
B) Hydrochloric acid and sodium hydroxide
C) Chlorine and oxygen
D) Nitric acid and sodium hydrogen carbonate
Answer:
B) Hydrochloric acid and sodium hydroxide
8. What colour would hydrochloric acid (pH = 1) turn universal indicator?
A) Orange
B) Purple
C) Yellow
D) Red
Answer:
D) Red
![]()
9. Which one of the following types of medicines is used for treating indigestion?
A) antibiotic
B) analgesic
C) antacid
D) antiseptic
Answer:
C) antacid
10. What gas is produced when magnesium is made to react with hydrochloric acid?
A) hydrogen
B) oxygen
C) carbon dioxide
D) no gas is produced
Answer:
A) hydrogen
11. Which of the following is the most accurate way of showing neutralization?
A) Acid + base → acid-base solution
B) Acid + base → salt + water
C) Acid + base → sodium chloride + hydrogen
D) Acid + base → neutral solution
Answer:
B) Acid + base → salt + water
10th Class Chemistry 1st Lesson Acids, Bases and Salts InText Questions and Answers
10th Class Chemistry Textbook Page No. 25
Question 1.
Is the substance present in antacid tablet acidic or basic?
A. The substance present in antacid tablet is basic.
Question 2.
What type of reaction takes place in stomach when an antacid tablet is consumed?
mrearx
A. Neutralization reaction takes place in stomach when an antacid tablet is consumed.
10th Class Chemistry Textbook Page No. 26
Question 3.
You are provided with three test tubes containing distilled water, an acid and a base solution respectively. If you are given only blue litmus paper, how do you identify the contents of each test tube?
Answer:
I know that acid turns blue litmus to red. With the help of this test I can find the acid. Distilled water and base don’t do so. Thus I identify each.
Question 4.
Which gas is usually liberated when an acid reacts with a metal? How will you test for the presence of this gas?
Answer:
Usually acids generate hydrogen gas on reacting with metals.
Test: When a burning splinder is brought near to the collected gas (H2), it puts off with a pop sound.
This test proves that the gas is H2.
![]()
Question 5.
A compound of a calcium reacts with dilute hydrochloric acid to produce effervescence. The gas evolved extinguishes a burning candle ; turns lime water milky. Write a balanced chemical equation for the reaction if one of the compounds formed is calcium chloride.
Answer:
Equation is : CaCO3 + 2 HCl → CaCl2 + CO2 + H2O
10th Class Chemistry Textbook Page No. 30
Question 6.
Why do HCl, HNO2 etc. show acidic characters in aqueous solutions while solutions of compounds like alcohol and glucose do not show acidic character?
Answer:
HCl, HNO3, etc. show acidic characters in aqueous solutions as they liberate H+ ions. But alcohol and glucose don’t liberate H+ ions. So, they do not show acidic character.
Question 7.
While diluting an acid, why is it recommended that the acid should be added to water and not water to the acid?
Answer:
1) If water is added to a concentrated acid, the heat generated may cause the mixture to splash out and cause burns.
2) The glass container may also break due to excessive local heating.
10th Class Chemistry Textbook Page No. 33
Question 8.
What will happen if the pH value of chemicals in our body increases?
Answer:
When pH value of chemicals in our body increases then the body will effect by some problems. They are
1) Digestion problems raise in the stomach.
2) pH changes as the cause of tooth decay.
![]()
Question 9.
Why do living organism have narrow pH range?
Answer:
Because increasing acidity is thought to have a range of possibly harmful consequences such as depressing metabolic rate and immune response in some organisms and causing coral bleaching
10th Class Chemistry 4th Lesson Acids, Bases and Salts Activities
Activity – 1
Question 1.
Observe the change in colour in each case and tabulate the results in the table.
Answer:
Procedure:
1) Collect the following samples from the science laboratory ;
i) Hydrochloric acid (HCl)
ii)Sulphuric acid (H2SO4)
iii) Nitric acid (HNO3)
iv) Acetic acid (CH3COOH)
v) Sodium hydroxide (NaOH)
vi) Calcium hydroxide[Ca(OH)2]
vii) Magnesium hydroxide [Mg(OH)2]
viii) Ammonium hydroxide(NH4OH)
ix) Potassium hydroxide (KOH)
2) Prepare dilute solutions of the respective substances.
3) Take four watch glasses.
4) Put one drop of the first solution in each one of them and test the solution as follows.
i) Dip the blue litmus paper in the first watch glass.
ii) Dip the red litmus paper in the second watch glass.
iii) Add a drop of methyl orange to the third watch glass.
iv) Add a drop of phenolphthalein to the fourth watch glass.
Observation :
Observe the respective colour changes and note down in the chart below.
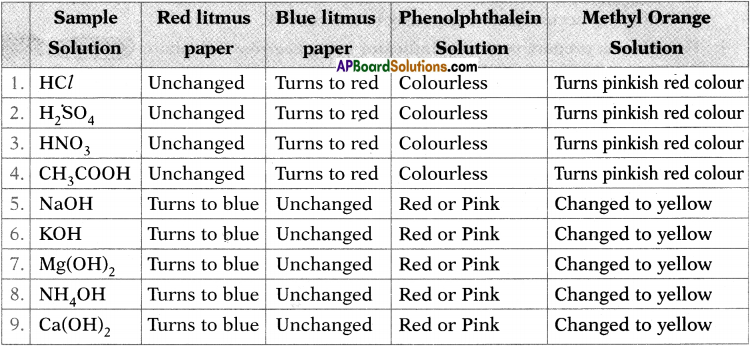
1. What do you conclude from the observations noted in the above table? (AS1)
Answer:
Conclusion : Acids turn blue litmus to red and bases turn red litmus to blue. Acids turn phenolphthalein to colourless and bases turn pink. Acids turn methyl orange to red and bases turn methyl orange to yellow.
2. Identify the above sample as acidic or basic solution. (AS4)
Answer:
Acids : HCl, H2SO4, HNO3, CH3COOH.
Bases : NaOH, KOH, Mg(OH)2, NH4OH, Ca(OH)2.
Activity -2
Question 2.
What are Olfactory indicators? Write an activity to prove them.
(OR)
What is the name given to a substance which identifies an acid or base by virtue of smell? Write an activity to prove the fact with an example.
Answer:
Olfactory Indicators : There are some substances whose odour changes in acidic or basic media. These are called olfactory indicators.
Activity :
Aim : To check the olfactory indicator.
Required materials :
1) Onions
2) Knife
3) Plastic bag
4) Clean clothes.
Procedure :
1) Take some onions and finely chop them.
2) Put the chopped onions in a plastic bag along with some clean cloth.
3) Tie up the bag tightly and keep it overnight in the fridge.
4) Then remove onions from fridge and add some base. We observe it loses its smell. Observation : Check the odour of the cloth strips.
Result: It is used as the basic indicator.
LAB ACTIVITY Reaction of Acids with metals
Question 3.
Write an experiment showing the reaction of acids with metals. (AS3)
(OR)
Ramu added acid to active metal then what is the gas which has been liberated. What are the apparatus required to prove the experiment. Write the experimental acitivity.
(OR)
Write the required material and experimental procedure for the experiment, “Hydrochloric acid reacts with ‘Zn’ pieces and liberates H2“.
Answer:
Aim : To show the reaction of acids with metals.
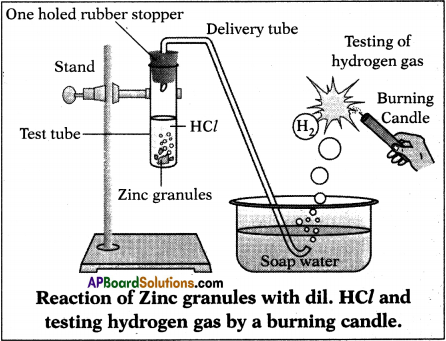
Required Materials :
- Test tube
- Delivery tube
- Glass trough
- Candle,
- Soap water
- Dil. HCl
- Zinc granules
- One holed rubber stopper
- Retard stand
Experimental procedure :
- Take some zinc granules in a test tube and arrange the test tube to the retart stand.
- Fix a delivery tube to the rubber stopper and immerse the second end of the delivery tube into the soap water.
- Add about 10 m/ of dilute hydrochloric acid to Zn granules and fix rubber stopper to the test tube.
- Evolved gas forms bubbles in soap water.
- Bring a lightened candle near to the gas bubbles. We can observe the burning of gas bubble with pop sound.
Result: We can conform that the evolved gas is hydrogen.
Chemical reaction:
Acid + Metal → Salt + Hydrogen
2Hcl(aq) + Zn(s) → Zncl2(aq) + H2(g)
Additional Experiment :
- Repeat the above experiment with H2SO4 and HNO3.
- We observe the same observation of the HCL experiment.
Conclusion : From the above activities we can conclude that when acid reacts with metal, H2 gas is evolved.
Activity – 3 Reaction of Bases with metals
Question 4.
Write an activity to show the reaction of bases with metals.
(OR)
Write an activity which proves certain bases produce hydrogen gas when they react with metals.
Answer:
Aim : To show the reaction of bases with metals.
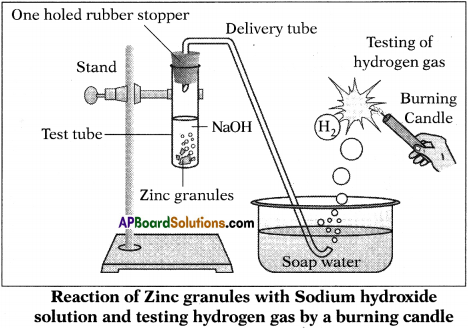
Required Materials :
- Test tube,
- Delivery tube
- Glass trough
- Candle
- Soap water
- Sodium hydroxide (NaOH) Solution
- Zinc granules
- One holed rubber stopper
Procedure :
- Set the apparatus as shown in figure.
- Take about 10 ml of dilute Sodium hydroxide (NaOH) solution in a test tube.
- Add a few granules of zinc metal to it.
- We will observe formation of gas bubbles on the surface of granules.
- The gas will pass through delivery tube evolved from soap solution as bubbles.
- Bring burning candle near the gas filled bubble.
- The gas in the bubble puts off the candle with pop sound.
Result: The evolved gas is hydrogen.
Chemical reaction :
Base + Metal → Salt + Hydrogen

Note : It is better to use cone. NaOH solution for this reaction.
Activity – 4 Reaction of carbonates and metal hydrogen carbonates with Acids
Question 5.
Write an activity to show that all metal carbonates and hydrogen carbonates react with acids to give a corresponding salt. (AS3)
Answer:
Aim : To show that all metal carbonates and hydrogen carbonates react with acids to give a corresponding salt.
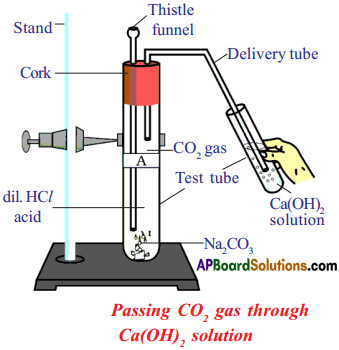
Required Materials :
- Two test tubes
- Sodium Carbonate (Na2CO3)
- Sodium Hydrogen Carbonate (NaHCO3)
- Two holed rubber stopper
- Thistle funnel
- Stand
- Dilute hydrochloric acid
- Delivery tube
- Calcium Carbonate (in a test tube)
Procedure :
- Take a test tube A with 0.5 gm of sodium carbonate.
- Close the test tube A with two holed rubber cork.
- Insert a thistle funnel through one hole and insert a delivery tube through the other hole.
- Pour 2 ml of dilute HC/ to the test tube A.
- Do the same as above with test tube B with sodium hydrogen carbonate.
Observation :
Carbon dioxide is released from test tube A and B. Passing CO2 gas through Ca(OH)2 solution
Chemical Reaction :
Na2CO3(s) + 2 HCl(aq) → 2 NaCl(aq) + CO2(g) + H2O(l)
Metal Carbonate + Acid → Salt + Carbon dioxide + Water
NaHCO3(s) + HCl(aq) → NaCl(aq) + CO2(g) + H2O(l)
Metal Hydrogen Carbonate + Acid → Salt + Carbon dioxide + Water
Result : All metal carbonates and hydrogen carbonates react with acids to give a corresponding salt.
Activity – 5 Neutralization reaction
Question 6.
Write an activity to find the change of colour in the reaction of an acid with a base (Neutralization) reaction. (AS3)
(OR)
Explain neutralization reaction with an activity.
Answer:
Aim : To test the change of colour in the reaction of an acid with a base.
Required Materials :
- 2 ml of dilute NaOH (Sodium Hydroxide) solution.
- Phenolphthalein indicator solution.
- dilute HCl (Hydrochloric) solution.
Procedure :
- Take about 2 ml of dilute NaOH solution in a test tube.
- Add two drops of phenolphthalein indicator solution.
Observation (i) :
- It turns to red or pink colour.
- It shows that NaOH is a base.
Experiment (1) : Add dilute HCl solution to the above solution drop by drop.
Observation (ii) : Pink colour disappears due to the reaction of NaOH (base) with HCl (acid).
Experiment (2) : Now add one or two drops of NaOH to the above mixture.
Observation (iii) : Pink colour reappears on adding NaOH.
NaOH + HCl → NaCl + H2O
base + acid → salt + water
Result: This reaction is called a neutralization reaction.
Activity – 6 Reaction of metallic oxides with acids
Question 7.
Write an activity to show that metal oxide reacts with acid is a neutralization. (AS3)
(OR)
How can you prove metallic acids are basic in nature?
Answer:
1) Take a small amount of copper oxide (CuO) in a beaker.
2) Add dilute HCl slowly while stirring.
3) Copper oxide dissolves in dilute HCl and the solution becomes blueish green colour due to the formation of copper (II) chloride.
Equation : Metal oxide + Acid → Salt + Water
Result: This reaction is same as the reaction of base with acid, (neutralization)
Question 8.
Write an activity to show that non-metallic oxide reacts with base is a neutralization.
Answer:
1) Take a small amount of calcium hydroxide (base).
2) Add CO2 into it.
3) Salt and water are produced.
Equation : Non-metallic oxide + Base → Salt + Water
Result: It is a neutralization reaction.
![]()
Question 9.
Repeat the activity – 7 using alkalis such as sodium hydroxide, calcium hydroxide solutions, etc. instead of acid solutions.
i) Does the bulb glow?
Answer:
Yes, the bulb will glow.
ii) What do you conclude from the results of this activity?
Answer:
Basic solutions are also good conductors of electricity due to released OH– ions.
iii) What happens to an acid or a base in aqueous solution?
Answer:
Acids produce H+ ions and bases produce OH– ions in aqueous solutions.
iv) Do acids produce ions only in aqueous solution?
Answer:
Yes.
Activity – 8
Question 10.
Do acids produce ions only in aqueous solution? Prove it. (AS3)
(OR)
Acids produce ions only in aqueous solution. Justify your answer with an activity.
Answer:
Procedure :
- Take about 1.0 g of solid NaCl in a clean and dry test tube.
- Add some concentrated sulphuric acid to the test tube. .
Observation :
- A gas comes out of the delivery tube.
- If we test the gas with dry and wet blue litmus paper, there is no change in colour.
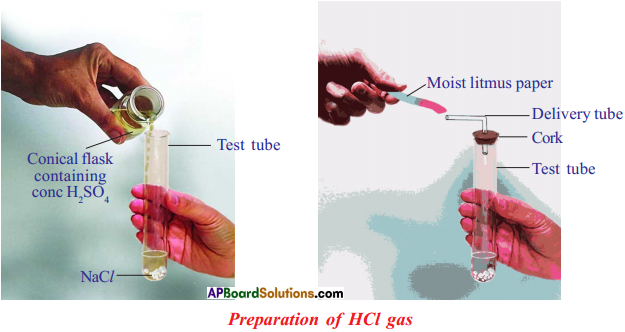
Chemical equation : 2 NaCl(s) + H2SO4(l) > 2 HCl + Na2SO4(s)
Conclusion :
- We can conclude that dry HCl gas (hydrogen chloride) is not an acid.
- Because we have noticed that there is no change in colour of dry litmus paper.
- But HCl aqueous solution is an acid because wet blue litmus paper turned into red.
Activity – 9 Reaction of water with acids or bases
Question 11.
Write an activity to show that dissolving of an acid in water is an exothermic process (or) endothermic process. (AS3)
(OR)
What do you observe when water is mixed with acid or base?
Answer:
Experiment :
- Take 10 ml water in a beaker.
- Add a few drops of concentrated H2SO4 to it and swirl the beaker slowly.
- Touch the base of the beaker.
- The base is hot.
- Do this experiment with other concentrated acids like HCl, HNO3 Result: This is an exothermic process called dilution.
Activity -10 Strength of acid or base
Question 12.
Write an activity to know whether the acid is strong or weak. (AS3)
Answer:
- Take dilute HCl in a beaker.
- Close it with a cardboard and introduce two different colour electrical wires through the holes made on it.
- Connect a bulb and make the connection as shown in the figure.
- Do the same replacing dilute HCl with dilute CH3COOH (acetic acid).

Observation :
The bulb glows brightly in HCl solution, while the bulb’s intensity is low in acetic acid solution.
Result:
More ions are present in HCl solution which is a strong acid than in CH3COOH solution which is a weak acid.
Activity – 11
Question 13.
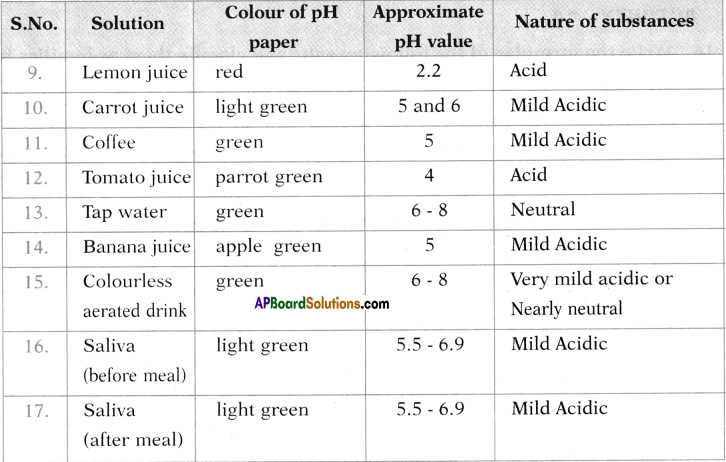
Test the pH value of solution given in table. Record your observations. What is the nature of each substance on the basis of your observations?
Answer:
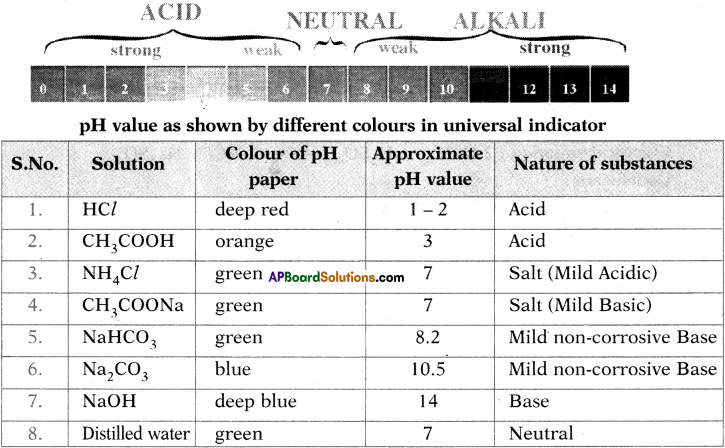
Activity – 12
Question 14.
Write an activity to check the colour change in dilute HCl and antacid solution in addition of methyl orange. (AS3)
Answer:
Procedure :
- Take dilute HCl in a beaker.
- Add two to three drops of methyl orange indicator to it.
- The solution colour turns to red.
- Now take the same solution and mix antacid tablet powder.
Observation :
Check the colour change.
Result:
The colour of the solution turns to light yellow.
Chemical equation:
2 HCl + Mg(OH)2 → MgCl2 + 2H2O
Activity – 13
Question 15.
How can we test the pH value of the soil? (AS3)
Answer:
- Take about 2g of soil in a test tube.
- Add 5 ml water to it.
- Shake it well.
- Filter the content.
- Collect the filtrate in a test tube.
- Add 2 drops of universal solution to it.
- Observe the colour.
- Compare the colour with strip colour on the bottle and find the pH value.
- In this way we can test the pH of the soil.
Activity – 14
Question 16.
Write the formulae of the following salts and classify them as families based on radicals.
Potassium Sulphate, Sodium Sulphate, Calcium Sulphate, Magnesium Sulphate, Copper Sulphate, Sodium Chloride, Sodium Nitrate, Sodium Carbonate and Ammonium Chloride. (AS4)
Answer:
| Name of the Salt | Formula |
| 1. Potassium Sulphate | K2SO4 |
| 2. Sodium Sulphate | Na2SO4 |
| 3. Calcium Sulphate | CaSO4 |
| 4. Magnesium Sulphate | MgSO4 |
| 5. Copper Sulphate | CuSO4 |
| 6. Sodium Chloride | NaCl |
| 7. Sodium Nitrate | NaNO3 |
| 8. Sodium Carbonate | Na2CO3 |
| 9. Ammonium Chloride | NH4Cl |
Sodium family : Na2SO4, NaCl, NaNO3, Na2CO3, etc.
Family of chloride salts : NaCl, NH4Cl, etc.
Family of sulphate salts : K2SO4, Na2SO4, CaSO4, MgSO4, CuSO4, etc.
Family of carbonate salts : Na2CO3 MgCO3 CaCO3, etc.
![]()
Question 17.
Identify the acids and bases from which they are obtained. (AS4)
Answer:
| Name of the Salt | Parent Acid | Parent Base |
| 1. Potassium Sulphate | Sulphuric Acid | Potassium Hydroxide |
| 2. Sodium Sulphate | Sulphuric Acid | Sodium Hydroxide |
| 3. Calcium Sulphate | Sulphuric Acid | Calcium Carbonate |
| 4. Magnesium Sulphate | Sulphuric Acid | Magnesium Hydroxide |
| 5. Copper Sulphate | Sulphuric Acid | Copper Hydroxide |
| 6. Sodium Chloride | Hydrochloric Acid | Sodium Hydroxide |
| 7. Sodium Nitrate | Nitric Acid | Sodium Hydroxide |
| 8. Sodium Carbonate | Carbonic Acid | Sodium Hydroxide |
| 9. Ammonium Chloride | Hydrochloric Acid | Ammonium Hydroxide |
Activity – 15 pH of Salts
Question 18.
Collect the salt samples like sodium chloride, aluminium chloride, copper sulphate, sodium acetate, ammonium chloride, sodium hydrogen carbonate and sodium carbonate. Dissolve them in distilled water. Check the action of these solutions with litmus papers. Find the pH using pH paper (universal indicator. Classify them into acidic, basic or neutral salts. Identify the acid and base used to form the above salts. Record your observations in table. (AS4)
Answer:
| Salt | pH | Acid | Base | Neutral |
| Sodium Chloride | 7 | – | – | ✓ |
| Aluminium Chloride | 7 | – | – | ✓ |
| Copper Sulphate | < 7 | ✓ | – | – |
| Sodium Acetate | > 7 | – | ✓ | – |
| Ammonium Chloride | < 7 | ✓ | – | – |
| Ammonium Chloride | > 7 | – | ✓ | – |
| Sodium Carbonate | > 7 | – | ✓ | – |