AP State Syllabus AP Board 8th Class Physical Science Solutions Chapter 9 Electrical Conductivity of Liquids Textbook Questions and Answers.
AP State Syllabus 8th Class Physical Science Solutions 9th Lesson Electrical Conductivity of Liquids
8th Class Physical Science 9th Lesson Electrical Conductivity of Liquids Textbook Questions and Answers
Improve Your Learning
Question 1.
Give examples for good solid conductors and liquid conductors.
Answer:
Examples for good conductors in solids:
Silver, copper, iron, graphite, etc.
Examples for good conductors in liquids:
Mercury, acid solutions (HCl, H2SO4), base solutions (NaOH, KOH), salt solutions (NaCl, KCl), etc.
![]()
Question 2.
Give examples for bad solid conductors and liquid conductors.
Answer:
Examples for poor conductors in solids:
Gold, platinum, etc.
Examples for bad conductors in liquids:
Distilled water, coconut oil, petrol, vegetable oil, kerosene, alcohol, etc.
Question 3.
What do you add to distilled water for making it to conduct electricity?
Answer:
Distilled water is poor conductor of electricity. In order to increase conductivity we should have to add either acid, base or salt. That will increase the conductivity of distilled water due to decomposition of these substances into constitute ions.
Question 4.
What is an electrolyte ?
Answer:
Electrolyte is a solution of substance through which current can pass.
Question 5.
Which energy is cause for glowing of bulb in electrolytic cell?
Answer:
Electrolytic cell is a device which converts chemical energy into electrical energy. So chemical energy stored in the cell is cause for glowing of bulb.
![]()
Question 6.
Write the uses of electroplating.
Answer:
Uses of electroplating:
- Metals like iron are coated with deposits of nickel or chromium to prevent rusting.
- Machinery parts are often chromium plated to protect them from corrosion and at the same time to give them good polish.
- Electroplating is also used in repairing worn out parts of machinery.
- Electroplating is also done for ornamentation and decoration purposes.
- Processed food items are preserved in tin coated iron cans by electroplating method.
- Zinc coated iron by electroplating method is used for bridges and in automobiles.
Question 7.
In case of a fire, before the fire men use the water, they shut off the main electrical supply for the area. Explain why they do this.
Answer:
Fire men use water to put out fire. Water containing dissolved salts is a good conductor of electricity. If fire men pour water on fire the electrical appliances near the fire may be wet if anybody touches those appliances they may have electric shock. In order to avoid people to get electric shock due to wet electrical appliances the fire men shut off electrical supply before they use water.
Question 8.
We get some items made from iron wire in which iron wire is coated with plastic. Is plastic coated by the process of electroplating? Why plastic cannot be coated on a metal by the process of electroplating?
Answer:
No. Plastic cannot be coated on a metal by using electroplating. The reason is plastic being a carbon polymer does not dissociate into ions. So it does not allow passage of current. So it does not act as an electrolyte. So electrolysis process is not possible with plastic which is main criteria for electroplating. So plastic cannot be coated on a metal by the process of electroplating.
Question 9.
Kavya observed that a discharged dry cell which kept in sunlight by her father for few hours got ability to glow LED. She got many doubts and questions to raise. Can you guess those questions or doubts? (OR)
Rohan observed that a discharged dry cell which kept in sunlight by his brother for few hours got ability to glow LED. He got many doubts and questions to raise. Guess the doubts or questions.
Answer:
- Why does the bulb glow?
- How does the dry cell charged?
- Which energy is useful in charging the discharged dry cell?
- Shall we use that energy to get rid from electrical power cut?
![]()
Question 10.
Explain the process of coating copper on iron key. Draw the circuit diagram.
(OR)
Conduct an experiment for coating on iron key with copper by electroplating method and prepare a note.
Lab Activity
Answer:
Aim: Coating an iron key with copper by electroplating method.
Required material: Copper plate of size 2 cm x 5 cm, crystals of copper sulphate, a key made by iron, glass beaker, water, sulphuric acid, battery cell and some connecting copper wires.
Procedure: Dissolve crystals of copper sulphate in pure water to prepare concentrated solution. Pour the solution in a beaker and add a few drops of dilute sulphuric acid to it.
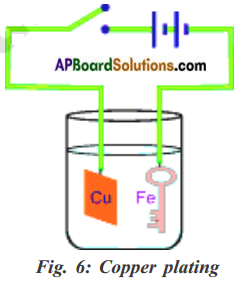
Tie one end of connecting copper wire to iron object to be coated with copper. Connect other end to the negative terminal of battery. Suspend the tied iron object into the copper sulphate solution. Suspend the copper plate into copper sulphate from positive end of the battery through a switch as shown in the above figure. Key and plate are a little away from each other. Put the switch on for about 10 minutes. Switch the circuit off and take the iron key out.
We can observe a red coating on iron key. The reason is when electric current is pass through copper solution, in which copper sulphate is present in the form of copper and sulphate ions. The copper ion gets drawn to the electrode connected to the negative terminal of the battery and deposited on it.
Question 11.
How do you appreciate the efforts of Luigi Galvani and Alessandro Volta in discovering a cell and making a stored electric energy available to human beings?
Answer:
We should have to thoroughly appreciate the efforts of Luigi Galvani and Alessandro for their discoveries for the development of mankind. Galvani and Volta completely change the life of human beings. Before that people generated electricity in different ways and conduct various experiments. However they faced one major problem which prevented them from understanding electricity in depth. They did not have a stable and permanent source of electricity. Galvani experimental conclusion was a revolution in science saying that all living beings contained electricity and it was the main source of life.
Volta proved it is possible to generate electricity if two different metals are placed in some liquids. Volta made first cell using zinc and copper dipped in sulphuric acid. This cell called Volta cell. Later dry cell was prepared. Now various appliances works with dry cells. So the efforts of Galvani and Volta should be appreciated by every generation for their contribution to electricity.
![]()
Question 12.
Collect the information and make a list of good conductors and bad conductors. Mow do you use this information in your daily life works?
Answer:
| Good conductors | Bad conductors |
| 1) Metals | 1) Wood |
| 2) Acid solutions | 2) Plastic |
| 3) Base solutions | 3) Diamond |
| 4) Salt solutions | 4) Distilled Water |
Applications in daily life: This information is very useful to us. Generally metal or good conductors of electricity are used in electrical appliances, electrical wires, fuse wires, etc. Whereas plastic is a bad conductor is used for electric insulators like gloves, handles of electrical appliances etc. to avoid electric shocks.
Question 13.
Make a battery from four lemons and test it with a LED in the circuit.
(OR)
Write how do you make a battery from four lemons and test it with a LED in the circuit in your laboratory.
Answer:
Take four lemons cut them into two pieces. Take one piece from each lemon and insert two copper wires and connect them in series and connect a LED and complete the circuit. The circuit is shown below.
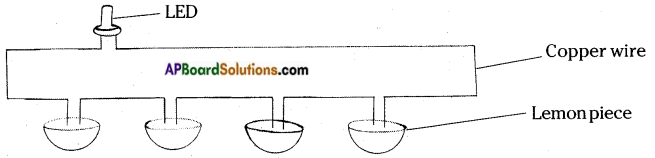
The bulb glows due to presence of current in the circuit. Here lemon juice acts as electrolyte and copper wires acts as electrolyte. So each lemon piece dipped with a copper wire acts as cell. These cells connected in series forms a battery.
Question 14.
Refer to the activity 3 in this chapter. Start with distilled water. The LED would not glow. Add two drops of some acid to distilled water and check the glow of LED. Add two more drops and check the intensity of the glow. Repeat the activity 5 to 6 times by adding 2 drops of the same acid each time. Do you see any difference in the intensity of glow with increasing acid content of water? What can be inferred from the above observations? Repeat the entire activity by taking a solution of baking soda and adding drops of it to distilled water instead of acid. Write differences and similarities.
Answer:
When we add two drops of acid (namely hydrochloric acid) to distilled water the LED will glow. If we further add the acid another two drops the intensity of glow increases. By repeating same activity 5 or 6 times we observe every time the glow of LED increases. The reason is acid dissociate into ions in aqueous solution. Which allows the passage of current. As the quantity of acid increases there will be more ions available for passage of electricity. So the intensity of glow increases.
We will observe the same result by adding baking soda but the intensity of glow is some what less when compared with addition of acid. The reason is baking soda is a weak base. So the dissociation is less when compared with acid. So less ions are available for passage of electricity. So the intensity of glow of LED is less when compared with acid.
![]()
Question 15.
In many of the activities in this chapter, we have used a tester made up of LED. Can we avoid LED and use something else as a tester Magnetic compass needle could be an alternative tester. We know that when we take a current carrying wire near magnetic compass needle, it shows deflection. Use this property to make a tester of magnetic compass needle. You may refer to the following figure.
Answer:
A magnetic compass wound with a copper wire is connected to one end of dry cell. The other end is connected to one of the two metal pins separated by a small gap in a rubber cap. The second pen is connected to the other end of dry cell to completed the circuit.

The magnetic needle deflects because whenever current pass through a wire it acts as a magnet is called magnetic effect of current.
8th Class Physical Science 9th Lesson Electrical Conductivity of Liquids InText Questions and Answers
Think and Discuss
8th Class Physical Science Textbook Page No. 123
Question 1.
Why some material allows electric current to pass through them and why some do not?
Answer:
Flow of charged particles constitutes current. So for the passage of current the material should have charged particles. All the materials do not have charged particles, e.g. Plastic, wood, diamond, etc. So only those material which have charged particles allow passage of current.
8th Class Physical Science Textbook Page No. 127
Question 2.
If a battery is packed in a box and if only two wires from two terminals are given out, how can we decide the positive and negative terminal of the battery?
Answer:
Insert the wires into a potato. A greenish spot is seen on potato at one of the wires. That wire behave like positive terminal and the other is negative terminal.
8th Class Physical Science Textbook Page No. 129
![]()
Question 3.
What is electrolysis?
Answer:
The dissociation of a solution of compound into constitute ions or elements by passing current is called electrolysis.
e.g. Electrolysis of water, which produce oxygen and hydrogen gases.
8th Class Physical Science 9th Lesson Electrical Conductivity of Liquids Activities
Activity – 1
Question 1.
Testing the material to know which allows electric current to pass through it.
Answer:
Take a torch bulb or LED (Light Emitting Diode), a dry cell, wooden sheet, two drawing pins, a key (safety pin) and pieces of connecting wires. Set up the electric circuit as shown in the figure.
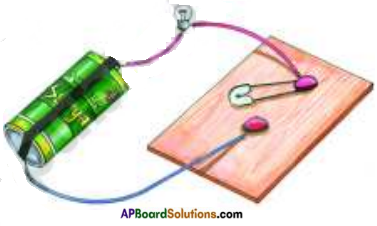
Place the key on drawing pin. The bulb begins to glow as soon as the key touches the drawing pin. Now replace the key by a nail.
Does the bulb glow ?
Yes, the bulb glows.
Repeat the activity using different types of materials instead of the nail, say a strip of paper, a piece of chalk, a drinking straw, a piece of plastic, a paper clip, a rubber eraser, etc.
Note in each case whether the bulb glows or not and enter your observations in table.
| Object | Material | Bulb glows Yes/No |
Good conductor/ bad or poor conductor |
| Nail | Iron | Yes | Good conductor |
| Eraser | Rubber | No | Bad or poor conductor |
| Paper | Cellulose | No | Bad or poor conductor |
| Chalk | Calcium carbonate | No | Bad or poor conductor |
| Straw | Plastic | No | Bad or poor conductor |
| Plastic | Plastic | No | Bad or poor conductor |
| Matchstick | Wood | No | Bad or poor conductor |
From the above activity, we conclude that some material allow electric current to pass through them. What we call them?
They are called good conductors of electricity.
The material that do not allow current to pass through them, what is the name given to those material?
They are named as bad or poor conductors of electricity.
![]()
Activity – 2
Question 2.
Testing the electric conductivity of liquids.
(OR)
Conduct an experiment for testing the electric conductivity of liquids.
Answer:
Take a LED, dry cell, metal pins, rubber cap of injection bottle and wires for making connections. Set up an electric
circuit shown in the figure.
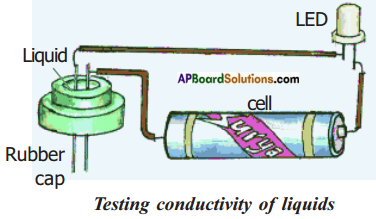
See that the two metal pins, pass through the cap and should have a very small gap (around 2 mm) between them so that the pins are fairly closer but not touching each other. The LED should not glow when pins are separated by small distance.
Now, join the free ends of pins together by pressing them for a moment and make sure that the LED glows. Release the pins they get separated and LED should not glow. This acts as a tester.
Fill the rubber cap with different liquids like distilled water that we drink, coconut oil, kerosene, lime juice, mustard oil, sugar solution, etc one after another and in each case check whether the LED glows or not. Note down your observations in table.
| Liquid | LED glows | Good conductor/ poor or bad conductor |
| Distilled water | No | Bad conductor |
| Drinking water | Yes | Good conductor |
| Coconut oil | No | Bad conductor |
| Lemon juice | Yes | Good conductor |
| Vinegar | Yes | Good conductor |
| Kerosene | No | Bad conductor |
| Vegetable oil | No | Bad conductor |
| Sugar solution | No | Bad conductor |
| Common salt solution | Yes | Good conductor |
| Milk | No | Bad conductor |
a) Why doesn’t the LED glow in all the cases? Or why doesn’t the LED remain off in all the cases?
Answer:
The LED doesn’t glow all the cases because when the liquid the two pins of tester allows electric current to pass through, the circuit is completed (closed) and the LED glows. On the other hand when the liquid does not allow the current to pass through, the circuit is incomplete (open) and the LED does not glow.
![]()
b) In the above activity, you may have observed that in all those cases where the LED glows, its brightness (intensity) is not the same. Sometimes it may be brighter and sometimes it may be relatively dimmer. Why is that so?
Answer:
The intensity of the glow of the LED depends on the flow of electric current through the circuit. Although a liquid may be a conductor, it may not allow current through it as easily as a metal does.
As a result although the circuit is completed and the LED glows, due to weak current in case of some of the liquids, the intensity of glow would be lower compared to other liquids.
Activity – 3
Question 3.
Transforming a poor electric conductor into a good conductor.
(OR)
Conduct an experiment for testing the electric conductivity of electrolyte.
Answer:
Take some amount of distilled water in three different containers. Dissolve small quantity of common salt in the water of first container. Dissolve the copper sulphate, lemon juice in second and third container respectively.
Use the tester that we used in activity 2, and repeat the activity 2. Note the observations in table.
| Material ‘ | Does the LED glow? Yes/No | Good conductor/bad conductor |
| Distilled water | No | Bad conductor |
| Distilled water + salt | Yes | Good conductor |
| Distilled water + CuSO4 | Yes | Good conductor |
| Distilled water + lemon juice | Yes | Good conductor |
a) From table what can we infer?
Answer:
Distilled water does not allow the electric current to pass. Water in its pure form is a bad conductor of electricity. But when water contains salts or acids, it allows a passage of electric current and turns into a good conductor of electricity.
b) Do you understand why you are advised not to touch electric appliances with wet hands?
Answer:
Water with salts is a good conductor of electricity and the current flowing through househt d electric appliances is very high. Therefore we should never touch the electrical appliances with wet hands.
Activity – 4
Question 4.
Testing the effect of electric current on potato.
Take a potato. Cut into two halves and take one half of it. Construct tester with LED bulb. Insert two copper wires of the tester into the potato. Leaving some distance (around 1 cm) between them.
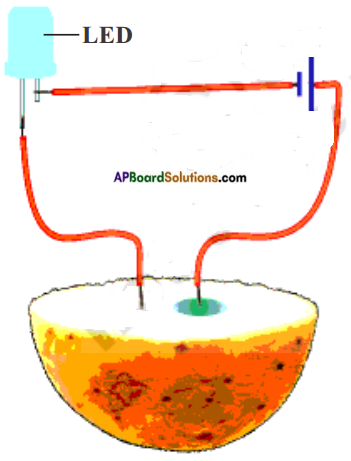
a) Dose the LED glow?
Answer:
Yes, the LED glows.
Leave the inserted wires for 20-30 minutes.
b) What do you observe the surface of the potato?
Answer:
A greenish blue spot is seen on the potato around the wire connected to the positive terminal of the battery.
But no such spot is seen around the other wire connected to the negative terminal. This greenish spot is due to chemical change in the potato.
![]()
c) What could be the cause behind this change?
Answer:
There is chemical change occurred in the potato.
d) Will other vegetables also show such an effect?
Answer:
Vegetable like carrot, beetroot, cucumber, radish, sweet potato show the chemical change there by the LED glows.
Activity – 5
Question 5.
Make your own cell.
(OR)
Draw the diagram of electrolytic cell and explain.
Collect two injection bottles. Cut two 5 cm long bits of thick copper wire. Use sandpaper to scrap about 1 cm of the coating off both ends of the wires.
Break open a exhausted dry cell and remove its outer metal covering (made of zinc). Cut two 2 mm wide and 5 cm long strips from this zinc plate. Insert the copper wires and zinc strips into the rubber caps of the injection bottles as shown in figure. Ensure that the copper wire and zinc strips do not touch each other.
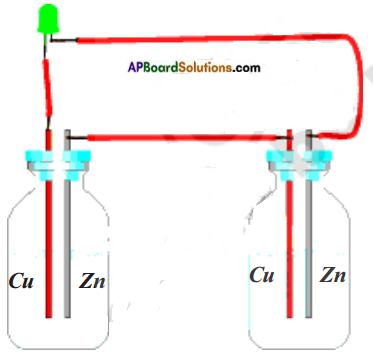
Now take a wire and connect the copper wire of one bottle with the zinc plate of the other bottle. Fill both bottles with dilute sulphuric acid. Carefully close the bottles with the caps in which copper wires and zinc strips are inserted. Your cell is ready.
How will you test it?
Take an LED. Attach two wires to its two terminals. Touch the wire from one terminal to the zinc plate and the wire from other terminal to the copper wire. Did the LED light up ? If not, change the connections vice-versa. Did the LED glow ?
Repeat the above activity using lemon juice, tamarind juice and tomato juice one by one instead of sulphuric acid to make cells.
a) What other liquids can be used to make the cell?
Answer:
Acid solutions.
b) Will detergent solution be useful?
Answer:
Yes, it is useful.
c) How does above cell function?
Answer:
After a few seconds of immersion of zinc and copper into dilute sulphuric acid, zinc slowly begins to dissolve in the sulphuric acid. We can see bubbles getting formed on the copper rod.
The current is passed from copper rod to zinc rod. Here chemical energy is converted into electric energy by electrolysis method.
![]()
d) Can you compare this cell with dry cell?
Answer:
In the above cell electrolyte is dilute sulphuric acid whereas in dry cell ammonium chloride paste is electrolyte. The electrodes in above cell is copper and zinc, whereas in dry cell it is graphite (carbon) and zinc.
e) Which is good one? Why?
Answer:
Dry cell is better than ordinary Volta cell. The reason dry cell does not have any fluids. So it is easy to carry dry cell compared with volta cell. So dry cell is better than volta cell.