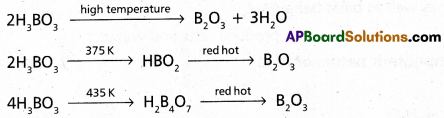
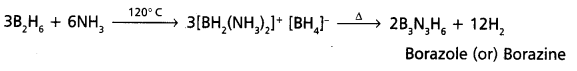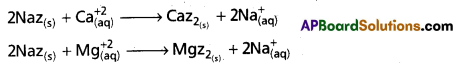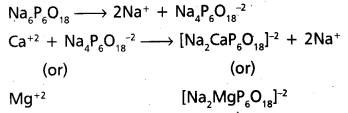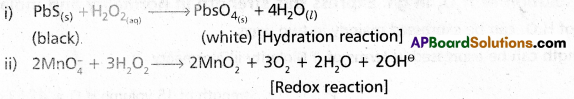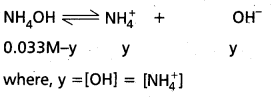Andhra Pradesh BIEAP AP Inter 1st Year Zoology Study Material Lesson 5 Locomotion and Reproduction in Protozoa Textbook Questions and Answers.
AP Inter 1st Year Zoology Study Material Lesson 5 Locomotion and Reproduction in Protozoa
Very Short Answer Type Questions
Question 1.
Draw a labeled diagram of the T.S of the flagellum.
Answer:
Question 2.
List any two differences between a flagellum and a cilium.
Answer:
| Flagellum | Cilium |
| 1. Flagellum helps in locomotion only. | 1. Cilium helps in locomotion and feeding and acts as sensory structures. |
| 2. Flagellum produces undular movement. | 2. Cilium produces pendular movement. |
| 3. Flagellum is about 150µ in length. | 3. Cilium is small in size 5-10µ in length. |
Question 3.
What are dynein arms? What is their significance?
Answer:
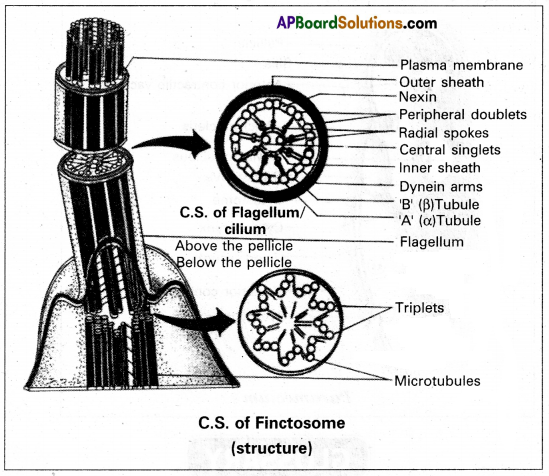
‘A’ tube of each peripheral doublet bears paired arms along its length called dynein arms made up of protein dynein.
The dynein arms of the ‘A’ tubule face the tubule ‘B’ of the adjacent doublet.
![]()
Question 4.
What is a kinety?
Answer:
In the ciliate protozoans, a longitudinal row of kinetosomes together with kinetodesmata constitute a unit called kinety.
Question 5.
Distinguish between synchronous and metachronous movements.
Answer:
Synchronous movement: Cilia in a transverse row beat simultaneously in one direction. It is called synchronous movement.
Metachronous movement: The sequential movement of cilia, in a longitudinal row, one after the other in one direction is called metachronous movement.
Question 6.
Why do we refer to the offspring, formed by the asexual method of reproduction, as a clone?
Answer:
As a result of the asexual method, the offsprings are, not only identical to one another but also exact copies of their parent. The term ‘clone’ is used to describe such morphologically and genetically similar individuals.
Question 7.
Distinguish between proter and opisthe.
Answer:
During transverse binary fission in Paramecium two daughters, individuals are formed. The anterior one is called proter and the posterior is called opisthe.
Question 8.
How is sexual reproduction advantageous in evolution?
Answer:
Sexual reproduction results the advantageous in evolution in genetic recombination occurs in sexual reproduction.
Question 9.
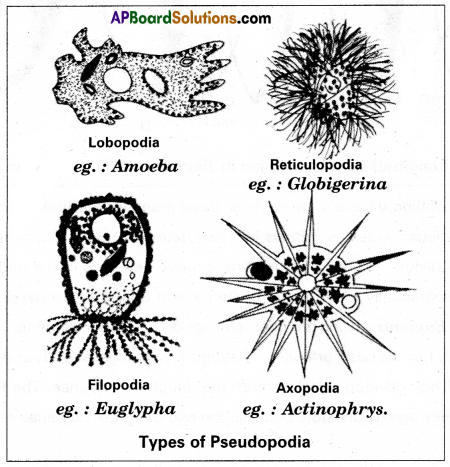
Distinguish between lobopodium and filopodium. Give an example to each of them.
Answer:
Lobopodium: The blunt and finger-like tubular pseudopodia containing both ectoplasm and endoplasm is called lobopodium.
Ex: Amoeba proteus
Filopodium: The slender filamentous pseudopodia with pointed tips, composed of only ectoplasm are called Filopodium.
Ex: Euglypha
Question 10.
Define conjugation with reference to ciliates. Give two examples.
Answer:
Conjugation is a temporary union between two senile ciliates that belong to two different ‘mating types’ for the exchange of nuclear material and its reorganization. – Wichterman.
Ex: Paramecium and Vorticella.
Short Answer Type Questions
Question 1.
Name the system that controls the fastest swimming movement of protozoans and write its components.
Answer:
Ciliary locomotion is the fastest swimming movement of protozoans, Hence, ciliates are the fastest protozoans.
Cilia are small hair-like structures found in ciliate protozoans like Paramecium.
Infraciliary system: It is located just below the pellicle in the ectoplasm of a ciliate. It includes kinetosomes, kinetodesmal fibrils, and kinetodesmata. The kinetosomes are present at the bases of cilia in transverse and longitudinal rows. The kinetodesmal fibrils are connected to the kinetosomes and run along the right side of each row of kinetosomes as a ‘cord of fibres’ called kinetodesmata. A longitudinal row of kinetosomes together with kinetodesmata constitute a unit called ‘kinety’.
All the kineties together form an infraciliary system, which is connected to a ‘motorium’, located near the cytopharynx. The infraciliary system and motorium form the ‘neuromotor system’ that controls and coordinates the movement of cilia.
![]()
Question 2.
Write the mechanism of bending the flagellum and explain effectively recovery strokes.
Answer:
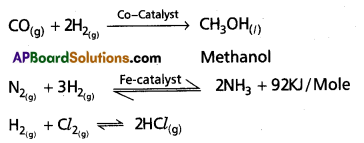
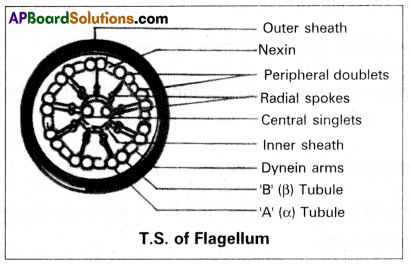
The bending movement of a flagellum is brought about by the sliding of microtubules past each other due to the functioning of ‘dynein arms’ utilizing ATP. A flagellum pushes the fluid medium at right angles to the surface of its attachment, by its bending movement.
Bending movement of flagella and cilia: Dynein arms show a complex cycle of movements using energy provided by ATP (dynein arms are the sites of ATPase activity in the cilia and flagella). The dynein arms of each doublet attach to an adjacent doublet and pull the neighbouring doublet. So the doublets slide past each other in opposite directions. The arms release and reattach a little further on the adjacent doublet and again ‘puli’. As the doublets of a flagellum or cilium are physically held in place by the radial spokes, the doublets cannot slide past much. Instead, they curve and cause the bending of flagellum or cilium. Such bending movements of flagella and cilia play an important role in flagellar and ciliary locomotion.
The flagellar movement of many organisms is a sidewise-lash which consists of two strokes namely the effective or propulsive stroke and the recovery stroke.
- Effective stroke: Flagellum becomes rigid and starts bending to one side beating against the water. This beating against water is at right angles to the body axis and the organism moves forwards.
- Recovery stroke: Flagellum becomes comparatively soft so as to offer the least resistance to water and moves back to its original position. It is called ‘recovery stroke’.
Question 3.
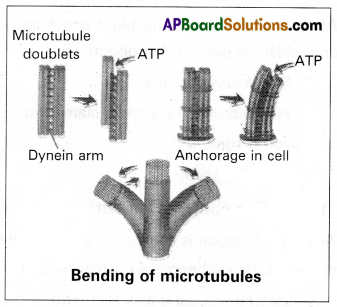
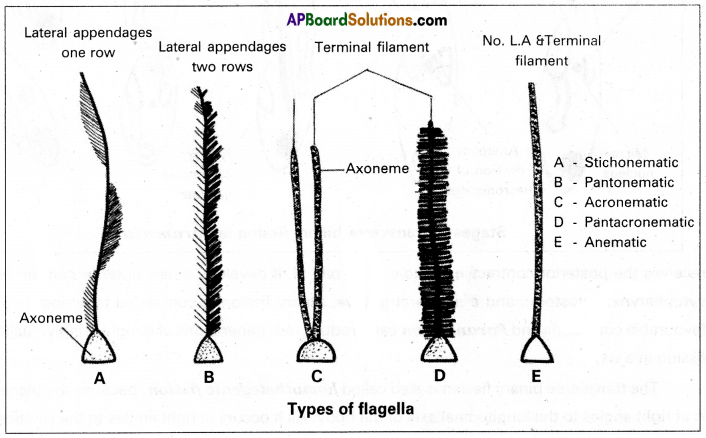
What are lateral appendages? Based on their presence and absence, write the various types of flagella giving atleast one example for each type.
Answer:
Lateral appendages: Some flagella bear one or two or many rows of short, lateral hair-like fibrils called lateral appendages. They are of two types namely ‘mastigonemes’ and ‘flimmers’.
Types of Flagella: Based on the presence or absence and/or the number of rows of lateral appendages, five types of flagella are recognized.
(a) Stichonematic: This flagellum bears one row of lateral appendages on the axoneme.
E.g. Euglena and Astasia.
(b) Pantonematic: This flagellum has two or more rows of lateral appendages on the axoneme.
E.g. Peranema and Monas.
(c) Acronematic: This type of flagellum does not bear lateral appendages and the terminal part of the axoneme is naked without the outer sheath at its tip.
E.g. Chlamydomonas and Polytoma
(d) Pantacronematic: This type of flagellum is provided with two or more rows of lateral appendages and the axoneme ends in a terminal naked filament.
E.g. Urceolus.
(e) Anematic or simple: In this type of flagellum, lateral appendages and terminal filament are absent. Hence, it is called anematic (a-no; nematic-threads)
E.g. Chilomonas and Gryptomonas.
![]()
Question 4.
Describe the process of transverse binary fission in paramecium.
Answer:
Transverse binary fission is performed by Paramecium. Binary fission is the most common method of sexual reproduction in protozoans. During favourable conditions, Paramecium stops feeding after attaining its maximum growth.
At first, the micronucleus divides by mitosis and the macronucleus divides into two daughter nuclei by amitosis. The oral groove disappears. After karyokinesis, a transverse constriction appears in the middle of the body, which deepens and divides the parent cell into two daughter individuals, the anterior proper and the posterior opisthe. The proper receives the anterior contractile vacuole, cytopharynx, and cytosome from its parent individual. It develops a posterior contractile vacuole and a new oral groove.
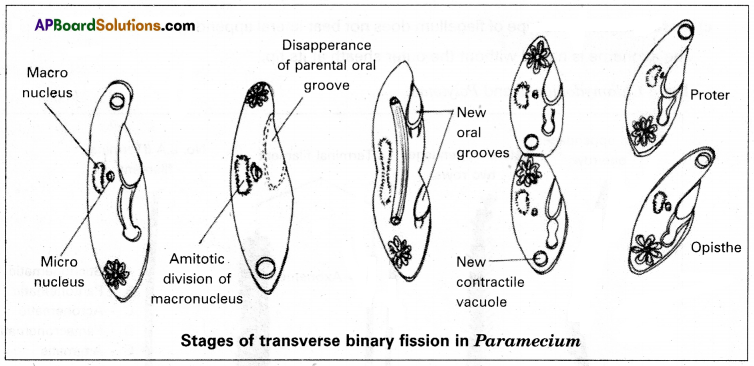
The opisthe receives the posterior contractile vacuole of its parent. It develops a new anterior contractile vacuole, cytopharynx, cytostome and a new oral groove. Binary fission is completed in almost two hours, in favourable conditions and Paramecium can produce four generations of daughter individuals by binary fission in a day. The transverse binary fission is also called homothetogenic fission because the plane of fission is at right angles to the longitudinal axis of the body. As it occurs at right angles to the kineties, it is also called perkinetal fission.
Question 5.
Describe the process of longitudinal binary fission in Euglena.
Answer:
Binary fission is the most common method of asexual reproduction in protozoans. Longitudinal binary fission is performed by Euglena. In this type of binary fission, the body divides into two halves longitudinally, hence called longitudinal binary fission.
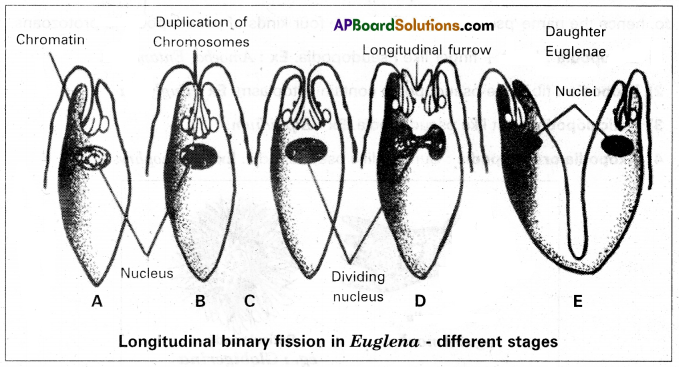
During the process of binary fission, the nucleus, basal granules, chromatophores, and cytoplasm undergo division. The nucleus divides by mitosis into two daughter nuclei. Then the kinetosomes and the chromatophores also divide. At first, a longitudinal groove develops in the middle of the anterior end. This groove extends gradually towards the posterior end until the two daughter individuals are separated. One daughter Euglena retains the parental flagella. The other daughter individual develops new flagella from the newly formed basal granules. The stigma, paraflagellar body, and contractile vacuole of the parent disappear. They develop afresh in both the daughter Euglenae. The longitudinal binary fission is known as symmetrogenic division because the two daughters Euglenae resemble each other like mirror images.
Question 6.
Write a short note on multiple fission.
Answer:
Multiple fission: It is the division Of the parent body into many smaller individuals (Multi-many; Fission- splitting). Normally multiple fission occurs during unfavourable conditions. During multiple fission, the nucleus first undergoes repeated mitotic divisions without cytokinesis. This causes the formation of many daughter nuclei. Then the cytoplasm also divides into as many bits as there are nuclei. Each cytoplasmic bit encircles one daughter nucleus. This results in the formation of many smaller individuals from a single-parent organism. There are different types of multiple fissions in protozoans such as Schizogony, malegametogony, sporogony in plasmodium, sporulation in Amoeba, etc.
![]()
Question 7.
Give an account of pseudopodia.
Answer:
Locomotion in protozoans is performed by cellular extensions such as pseudopodia found in rhizopods organisms. The pseudopodia are temporary extensions of cytoplasm that develop in the direction of the movement. These temporary structures are useful to move on the substratum as our legs do, hence the name ‘pseudopodia’. There are four kinds of pseudopodia in protozoans.
- Lobopodia: blunt + finger like Pseudopodia. Ex: Amoeba, Entamoeba.
- Filopodia: fiber like pseudopodia contain ectoplasm. Ex: Euglypha.
- Reticulopodia: net like pseudopodia. Ex: Elphidium.
- Axopodia or heliopodia: Sun ray-like pseudopodia. Ex: Actinophiys.
Question 8.
Give an account of the ultrastructure of an axoneme.
Answer:
Ultrastructure of flagellum: The axial filament or axoneme shows 9+2 organisation under the electron microscope. Two central singlets are enclosed by a fibrous inner sheath. Nine peripheral doublets form a cylinder between the inner sheath and the outer sheath. The “A” microtubule of each doublet is connected to the inner sheath by radial spokes. It also has pairs of arms all along the length and is directed towards the neighbouring doublet.
These arms are made of a protein called dynein. These arms create the sliding force. The peripheral doublets are surrounded by an outer membranous sheath called a protoplasmic sheath, which is the extension of the plasma membrane. Some flagella bear lateral appendages called flimmers or mastigonemes along the length of the axoneme above the level of the pellicle. Each flagellum arises from a basal granule that lies below the cell surface in the ectoplasm.
Question 9.
Draw a neat labelled diagram of Euglena.
Answer:
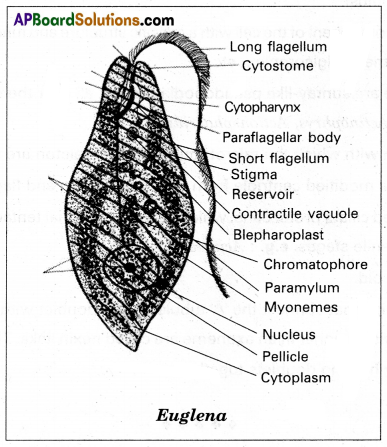
Question 10.
Draw a neat diagram of paramecium and label its important structures/components.
Answer:
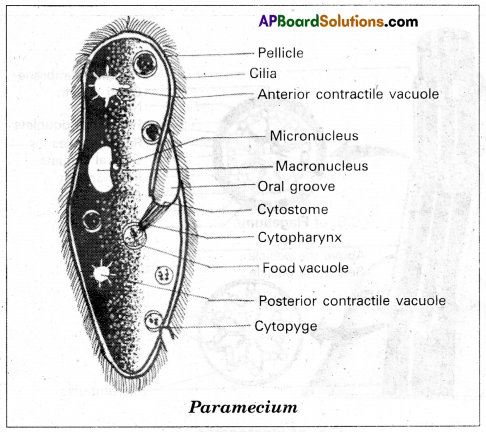







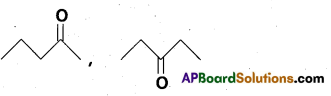
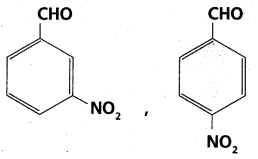
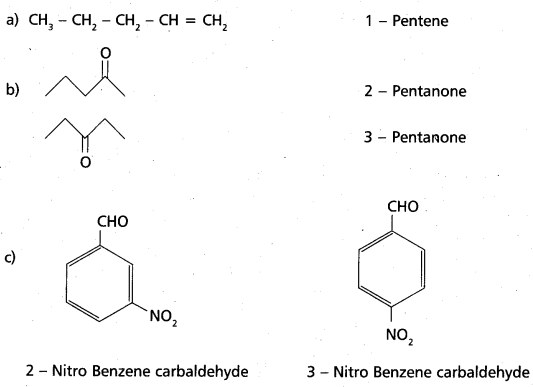



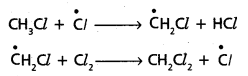
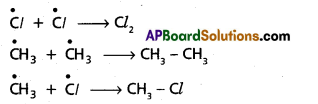
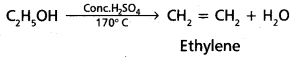
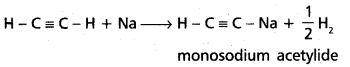
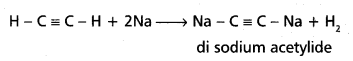
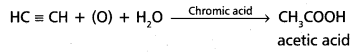
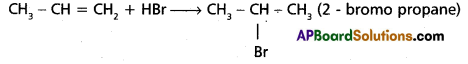
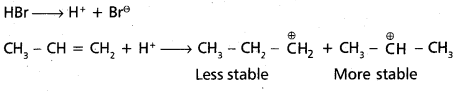
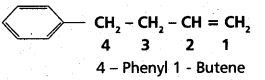
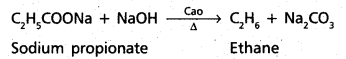
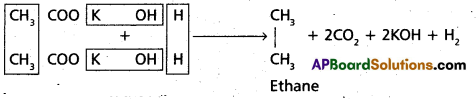
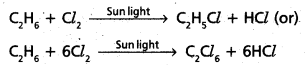

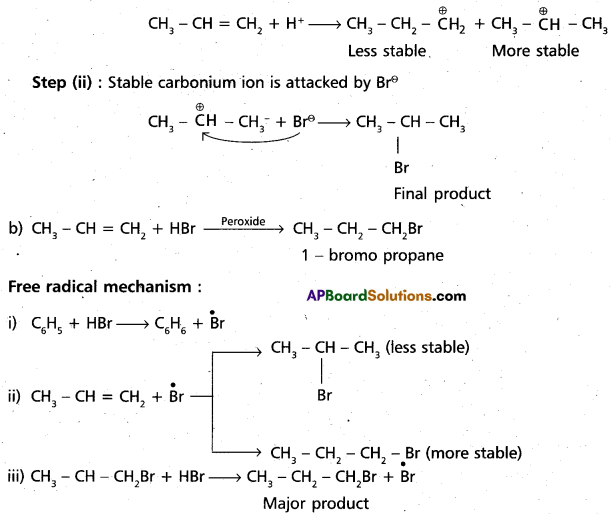
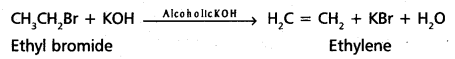
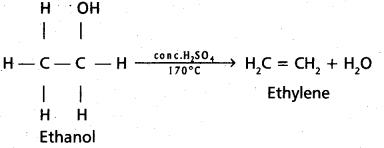
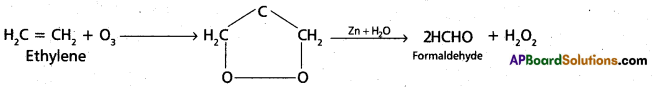
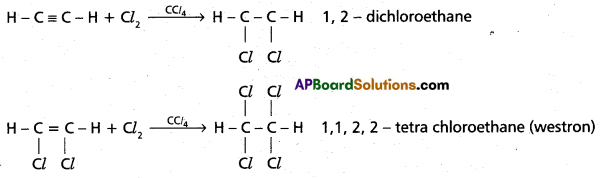
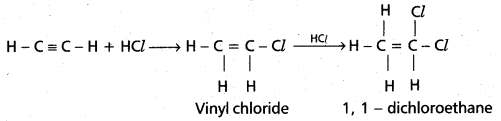


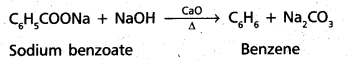
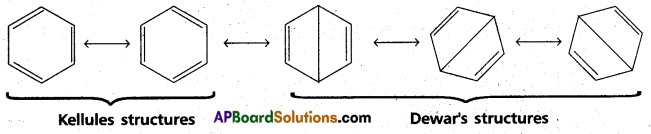
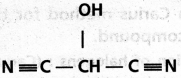
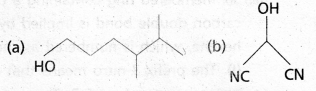
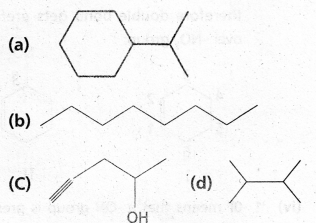
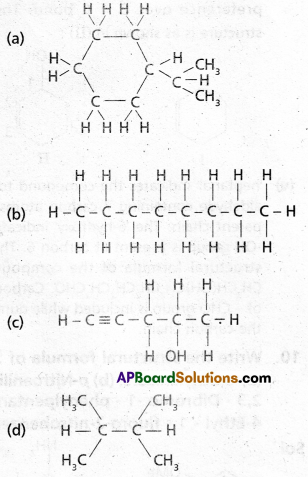
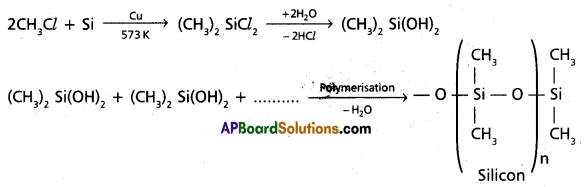
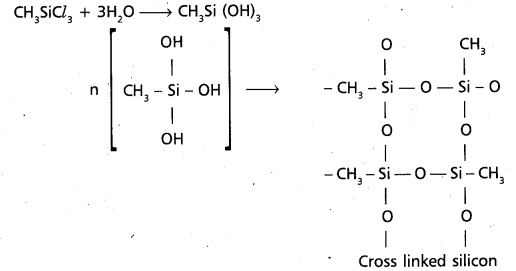
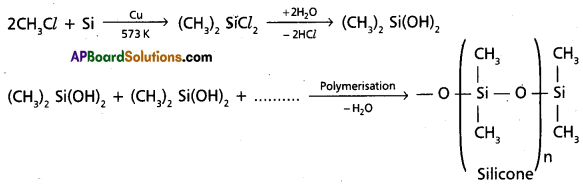
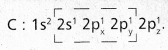
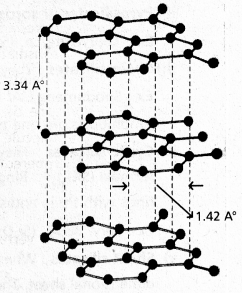
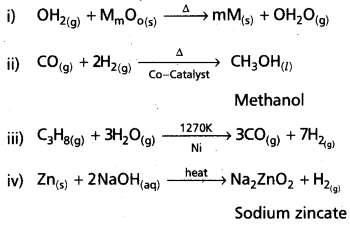
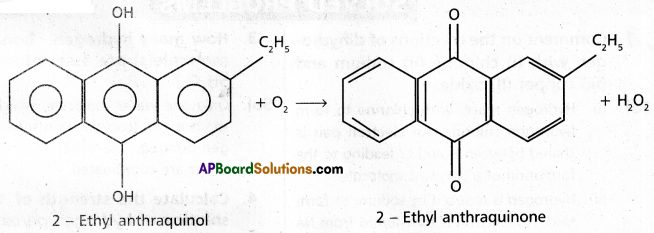
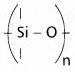 chains in which alkyl or phenyl groups occupy the remaining bonding positions on each silicon. They are hydrophobic (water repellant) in nature.
chains in which alkyl or phenyl groups occupy the remaining bonding positions on each silicon. They are hydrophobic (water repellant) in nature.