Andhra Pradesh BIEAP AP Inter 1st Year Chemistry Study Material 8th Lesson Hydrogen and its Compounds Textbook Questions and Answers.
AP Inter 1st Year Chemistry Study Material 8th Lesson Hydrogen and its Compounds
Very Short Answer Questions
Question 1.
The three isotopes of hydrogen differ in their rates of reaction. Give the reasons.
Answer:
The three isotopes of hydrogen differ in their rates of reactions due to their different enthalpies of bond dissociation.
Question 2.
Why is dihydrogen used in the welding of high melting metals?
Answer:
Dihydrogen is used in welding high melting metals because the atomic hydrogens which are formed by the dissociation of dihydrogen are recombined on the surface of the metal to be welded and produce a high temperature of 4000 K.
![]()
Question 3.
Describe one method of producing high purity hydrogen.
Answer:
Highly pure hydrogen is obtained by the electrolysis of hot aq.Ba(OH)2 solution between nickel electrodes. The purity of hydrogen is about more than 99.95%.
Question 4.
Explain the term “SYNGAS”.
Answer:
The mixture of CO and H2 which is used for the synthesis of methanol and a number of hydrocar-bons is called “SYNGAS”. It is also called as synthesis gas.
Preparation :
![]()
This reaction is called ‘Coal gasification’.
Question 5.
What is meant by coal gasification ? Explain with relevant, balanced equation.
Answer:
The process of producing synthesis gas (SYNGAS) by using coal at 1270K temperature is called coal gasification.
Balanced equation :
![]()
Question 6.
Define the term Hydride. How many categories of hydrides are known ? Name them.
Answer:
The binary compounds of hydrogen formed by the other elements except noble gases are called hydrides.
Hydrides are categorised into three types.
- Ionic hydrides
- Covalent hydrides
- Metallic hydrides
![]()
Question 7.
The unusual property of water in condensed phase leads to its high heat of vapourization. What is that property ?
Answer:
In water inter molecular hydrogen bonding is present. Due to this unusual property water has high Freezing point. Boiling point and high heat of vapourization.
Question 8.
During photosynthesis, water is oxidised to O2. Which element is reduced ?
Answer:
During photosynthesis, the element reduced is carbon.
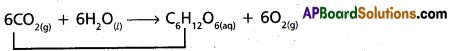
Oxidation state change from +4 to 0 [Reduction]
Question 9.
What do you mean by autoprotolysis ? Give the equation to represent the autoprotolysis of water.
Answer:
Water has the ability to behave as an acid as well as base. It behaves as an amphoteric substance. The self-ionising property of water is called auto protolysis.
The equation that represent the auto protolysis of water is as follows.
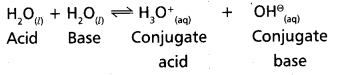
![]()
Question 10.
Water behaves as an amphoteric substance in the Bronsted sense. How do you explain ?
Answer:
According to Bronsted proton donor is acid and acceptor is base. Water has the ability to behave as an acid as well as base. So it is an amphoteric substance.
Eg :
- Water acts as acid with ammonia.
H2O(l) + NH3(aq) ⇌ OHΘ(aq) + \(\mathrm{NH}_{4 \text { (aq) }}^{\oplus}\)
Here water is proton donor. - Water acts as base with H2S.
H2O(l) + H2S(aq) ⇌ H3O⊕(aq) + HSΘ(aq)
Here water is proton acceptor.
Short Answer Questions
Question 1.
The boiling points of NH3 ; H2O and HF are higher than those of hydrides of the subsequent members of the group. Give your reasons.
Answer:
The boiling points of NH3, H2O and HF are higher than those of hydrides of the subsequent mem-bers of the group.
Reasons :
- NH3, H2O and HF are electron rich hydrides and these have 1, 2 and 3 lone pairs respectively.
- Due to the presence of lonepairs on the high electronegative elements results in the formation of hydrogen bond [i.e. intermolecular hydrogen bonding].
- Due to the formation of hydrogen bond association of molecules takes place. Hence these hydrides has high boiling points.
Question 2.
Discuss the position of hydrogen in the periodic table on the basis of its electronic configuration.
Answer:
Hydrogen is the simplest element with one electron and proton. Its electronic configuration is 1s. The configuration is responsible for its dual nature. It behaves both like alkali metals and halogens. So it can be placed along with alkali metals (IA) or along with halogens (VIIA).
Points in support of placing it in IA group :
a) Like alkali metals hydrogen also has a single electron in the outer shell 1s1,
b) Its ability to form hydrated unipositive ion, H+ (aq).
c) It is quite reasonable to start the periodic table with an element having the least atomic number (Z = 1).
Points in support of placing it in VIIA group :
a) Hydrogen is a gas like fluorine or chlorine.
b) It can form diatomic molecule like halogens.
c) It has a tendency of gaining an electron and attains a stable electronic configuration of He forming H+ ion like halogens.
At the same time it should be noted that hydrogen has not such a great tendency to lose electron like alkali metals and gain an electron like halogens. In view of this it is difficult to assign any definite position to hydrogen. Sometimes it is placed in IA group and sometime with VIIA group.
![]()
Question 3.
How is the electronic configuration of hydrogen suitable for its chemical reactions ?
Answer:
Electronic configuration of hydrogen is 1s1.
- The atomic hydrogen obtained from dihydrogen by the treatment with UV rays does combine with allmost all the elements.
- This atomic hydrogen successfully complete the reactions,
a) By the loss of one electron to give H⊕.
b) By the gain of one electron to form HΘ and
c) By the sharing of electrons to form a single covalent bond.
Eg : 1) H2(g) + F2(g) → 2HF(g)
2) 2Li(s) + H2(g) → 2LiH
Question 4.
What happens when dihydrogen reacts with
a) Chlorine and
b) Sodium metal. Explain.
Answer:
a) Reaction of dihydrogen with chlorine : Hydrogen reacts with chlorine to form hydrogen chloride gas. This reaction occurs in presence of sun light.
![]()
b) Reaction of dihydrogen with sodium metal : Hydrogen reacts with highly reactive metal like sodium and forms sodium hydride. The reaction occur at a high temperature.
2Na(s) + H2(g) → 2NaH(s)
Question 5.
Write a note on heavy water.
Answer:
Deuterium oxide (D2O) is known as heavy water.
Peparation : Heavy water is obtained by the exhaustive electrolysis of water.
The physical properties like molecular mass, melting point, Boiling point etc., of D2O are heavier than watet. But dielectric constant and solubility are low for heavy water when compared to water.
Chemical properties :
a) Heavy water reacts with calcium carbide and forms Deuteroacetylene.
CaC2 + 2D2O → C2D2 + Ca(OD)2
b) Heavy water reacts with sulphur trioxide and forms Deutero sulphuric acid.
SO3 + D2O → D2SO4
c) Heavy water reacts with Aluminium carbide and forms Deutero methane.
Al4C3 + 12D2O → 3CD4 + 4Al (OD)3
Uses :
- It is used as a moderator in nuclear reactors to decrease the speed of neutrons.
- It is used to study the reaction mechanism in exchange reactions.
- It is used for the preparation of deuterium and deuterium compounds.
![]()
Question 6.
Name the isotopes of hydrogen. What is the ratio of masses of these isotopes ?
Answer:
Hydrogen has three isotopes.
- Protium (1H1)
- Deuterium (1H2 (or) D)
- Tritium (1H3 or T)
Tritium is radioactive isotope.
- The ratio of masses of these isotopes is 1 : 2 : 3 respectively for protium, deuterium and Tritium.
- Protium has no neutrons, deuterium has one neutron and tritium has two neutrons.
Question 7.
What is water – gas shift reaction? How can the production of dihydrogen be increased by this reaction?
Answer:
The mixture of CO and H2 is called water gas. It is also called ‘Syngas’.
Water-gas shift reaction : When carbon monoxide of the syngas mixture reacted in presence of Iron chromate as catalyst then the reaction is called as water-gas shift reaction.
By using this reaction dihydrogen production can be increased.
![]()
In this reaction CO2 gas is removed by using sodiumarsenite solution by scrubbing.
Question 8.
Complete and balance the following reactions :
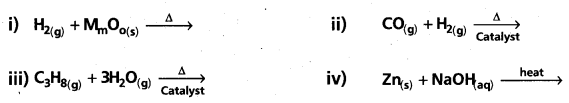
Answer:
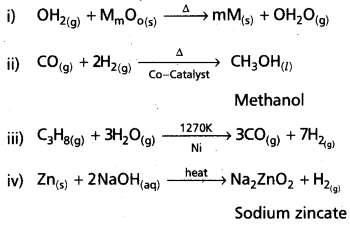
![]()
Question 9.
What is the nature of the hydrides formed by elements of 13 group ?
Answer:
Group 13 elements are belongs to p-block of periodic table.
Generally p-block elements forms covalent (or) molecular hydrides.
These molecular hydrides are further classified into 3 types
- Electron deficient hydrides
- Electron – precise hydrides
- Electron – rich hydrides
Group – 13 elements forms electron deficient hydrides. Electron deficient hydrides are the molecular hydrides which have less no.of electrons to write the lewis structure.
Eg : Diborane (B2H6)
These hydrides acts as Lewis acids. Lewis acids accepts electron pairs and forms co-ordinate covalent bonds with donors.
Question 10.
Discuss the principle and the method of softening of hard water by synthetic, ion- exchange resins.
Answer:
Synthetic ion – exchange resins method :
- In present days, this method is mostly used for softening the hard water by using synthetic resins.
- This method is more useful than zeolite (or) permutit process.
Principle : Formation of de-ionised water (or) de mineralised water by passing the water successively through cation exchange and anion exchange resins.
De-ionised water means, water which is free from all soluble mineral salts.
Process : The de-ionization of water can be done in two steps by this process.
Step – I: Cation exchange process.
Step – II: Anion exchange process.
Step – I: Cation exchange process
In this process synthetic resins used are – So3H group containing large organic molecule. (R-SO3H)
Here R = organic group (or) resin anion.
- At first the synthetic resin converted to RNa by reacting it with NaCl.
- This resin i.e; RNa exchanges Ca+2 and Mg+2 ions of hard water and the softening of water takes place.
2RNa(s) + \(\mathrm{M}_{(\mathrm{aq})}^{+2}\) → R2M(s) + 2\(\mathrm{Na}_{(\mathrm{aq})}^{+}\) - The resin can be regenerated by using aq.NaCl solution.
- In this step H+ ions exchanges Na+, Ca+2, Mg+2 and proton formation takes place.
2RH(s) + \(\mathrm{M}_{(\mathrm{aq})}^{+2}\) ⇌ MR2(s) + 2\(\mathrm{H}_{(\mathrm{aq})}^{+}\)
Step – II: Anion exchange process :
- In this process resins used are RNH3OH i.e., a basic compound.
- The OH– ions of resin exchanges the’anions Cl–, SO4-2, HCO3– etc., and OHΘ ion formation takes place.
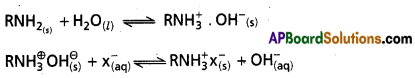
- The H+ ions and OH– ions obtained in the two steps are get neutralised to form de-ionised water.
\(\mathrm{H}_{(\mathrm{aq})}^{+}+\mathrm{OH}_{(\mathrm{aq})}^{-} \longrightarrow \mathrm{H}_2 \mathrm{O}_{(l)}\) - The cation exchange resins and anion exchange resins are regenerated by treatment with dil.acid and base respectively.
![]()
Question 11.
Write a few lines on the utility of hydrogen as a fuel. [Mar. 13]
Answer:
Hydrogen as a fuel :
The heat of combustion of hydrogen is high i.e about 242kj/mole. Hence hydrogen is used as industrial fuel.
- The energy released by the combustion of dihydrogen is more than the petrol (3 times).
- Hydrogen is major constituent in fuel gases like coal gas and water gas.
- Hydrogen is also used in fuel cells for the generation of electric power.
- 5% dihydrogen is used in CNG for running four-wheeler vehicles:
- By hydrogen economy principle the storage and transportation of energy in the form of liquid (or) gaseous state. Here energy is transmitted in the form of dihydrogen and not as electric power.
Question 12.
A 1% solution of H2O2 is provided to you. What steps do you take to prepare pure H2O2 from it ?
Answer:
To obtain pure H2O2 from the provided 1% H2O2 the following steps are involved.
Step -1:
The provided 1% H2O2 solution is carefully evoparated on a water bath under reduced pressure by distillation.
Here approximately 30% H2O2 solution is obtained.
Step – II :
The obtained solution in the above step is heated in a distillation flask at a low pressure of 15mm. Here approximately 85% H2O2 solution is obtained.
Step – III :
The above sample (obtained in the step – II) crystallised by freezing and pure H2O2 is obtained (≅ 100%).
![]()
Question 13.
Mention any three uses of H2O2 in modern times.
Answer:
Uses of H2O2 in modern times :
- H2O2 is used in modern times in Green chemistry to control the pollution.
Eg : It is used in the treatment of domestic and industrial effluents and in the oxidation of cyanides. - H2O2 is used in manufacturing of Sodium perborate and sodium per carbonates. These are used in high quality detergents.
- H2O2 is used in certain food products and in certain pharmaceuticals.
- H2O2 is used as bleaching agent to bleach paper pulp, leather etc.
- H2O2 is used as an antiseptic and in hair bleach.
Long Answer Questions
Question 1.
Write an essay on the commercial preparation of dihydrogen. Give balanced equations.
Answer:
Commercial methods of preparation of dihydrogen
i) From Hydrocarbons : When hydrocarbons undergo reaction with steam at high temperatures in presence of catalyst liberates hydrogen gas.
Eg : C3H 8(g) + 3H2°(g) ![]() 3CO + 7H2
3CO + 7H2
ii) By the electrolysis of water : Acidified (or) alkaline water undergo electrolysis using platinum electrodes liberates hydrogen gas.
![]()
Highly pure hydrogen is liberated by the electrolysis of hot aq. Ba(OH)2 by using ‘Ni’ electrodes.
iii) By Nelson’s Process : Hydrogen gas is obtained as a Byproduct by the electrolysis of brine solution. This process is mainly used for the manufacturing of NaOH, \(\mathrm{Cl}_2^{-}\) gas.
Cell Reactions : –
2NaCl → 2Na+ + 2Cl–
2Cl– → Cl2 + 2e– (Anode)
2H2O + 2e– → H2 + 2OH– (Cathode)
2Na+ + 2OH– → 2 NaoH
iv) By water gas shift reaction : Water gas is the mixture of CO and H2. It is also called as syngas. The process of producing syngas from coal is called as coal gasification.
![]()
The dihydrogen production can be increased by the reaction of steam with syngas in presence of catalyst.
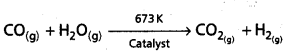
This reaction is called as water gas shift reaction.
![]()
Question 2.
Illustrate the chemistry of dihydrogen by its reaction with
i) N2
ii) Metal ions and metal oxides and
iii) Organic compounds. How is dihydrogen used in the manufacture of chemicals ?
Answer:
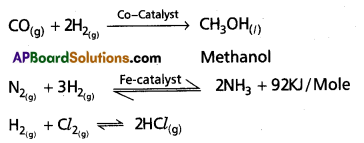
i) Reaction with N2: Dihydrogen reacts with nitrogen in presence of iron catalyst to form ammonia.
![]()
This process is called as Haber’s process. Here temperature used is around 700K and pressure used is 200 atm.
ii) a) Reaction with metal ions : Hydrogen reduces metal ions in aqueous solution into metals.
H2(g) + \(\mathrm{Pd}_{(\mathrm{aq})}^{+2}\) → Pd(s) + \(2 \mathrm{H}_{\text {(aq) }}^{+}\)
b) Reaction with metal oxides : Hydrogen reduces metal oxides into metals.
WO3 + 3H2 → W + 3H2O
iii) Reaction with organic compounds :
a) Vegetable oils undergoes hydrogenation in presence of ‘Ni’ catalyst and forms vanaspathi.
b) Alkenes undergo hydro formylation and forms aldehydes. These aldehydes undergo reduction to form alcohols.
CH2 = CH2 + CO + H2 → CH3 – CH2CHO (Aldehyde)
CH3CH2CHO + H2 → CH3CH2CH2OH (Alcohol )
Use of Dihydrogen in the manufacture of chemicals
Dihydrogen is used in the manufacturing of industrial cherfiicals like methanol, ammonia, hydrogen chloride etc.
Question 3.
Explain, with suitable examples, the following :
- electron deficient
- electron – precise and
- electron – rich hydrides.
Answer:
Molecular hydrides (or) covalent hydrides are formed by the p-block elements.
These molecular hydrides are divided into three types.
- Electron deficient hydrides
- Electron – precise hydrides
- Electron – rich hydrides
1) Electron – deficient hydrides : These are the molecular hydrides in which the available no.of valency electrons is less than the number-required for normal covalent bond formation.
(or) .
These are the molecular hydrides in which the available valency electrons are lessthan the required for writting the Lewis structure of the molecule.
Eg : (AlH3)n, B2H6 etc.
These hydrides acts as Lewis acids i.e. electron pair acceptors. These forms dative bond with donors.
2) Electron – precise hydrides : These are the molecular hydrides in which all the valency electrons of the central atom are involved in bond formation.
(or)
These are the molecular hydrides which contains the required no.of valency electrons to write the Lewis structure of the molecule.
Eg : Group 14 elements forms this type of hydrides CH4, C2H6 etc., are examples of this type.
These have tetrahedral geometry.
3) Electron – rich hydrides :
These are the molecular hydrides in which the valency electrons on the central atom are more than that are required for bond formation.
(or)
- These are the molecular hydrides in which the available valency electrons are more than the required for writting the Lewis structure of the molecule.
- These hydrides contains lone pairs on central atoms.
Eg :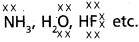
These hydrides have high boiling points than those of the hydrides of the subsequent members of group because of hydrogen bond formation.
![]()
Question 4.
Write in brief on
i) ionic hydrides
ii) interstitial hydrides.
Answer:
i) Ionic hydrides : These are also called as saline hydrides (or) salt like hydrides.
* These are the hydrides formed by combining di hydrogen with s-block elements (Electro posi-tive elements).
* These are stoichiometric compounds.
Eg : LiH, NaH, CaH2 etc.
NaH is formed by the direct union of Na and H2
![]()
Physical properties :
- These hydrides are crystalline.
- These hydrides are non volatile and non – conducting in solidstate.
- These conducts electricity in moltenstate.
- These have high melting points.
Chemical properties :
These hydrides on electrolysis, liberates dihydrogen gas at anode.
2H– → H2(g) + 2e– [Anode]
Lithium hydride is used in the synthesis of other useful hydrides like LiAlH4 and LiBH4.
8LiH + Al2CL6 → 2LiAlH4 + 6LiCl
2LiH + B2H6 → 2LiBH4
2NaH + B2H6 → 2NaBH4
These hydrides react with acids and water vigorously and liberate dihydrogen.
LiH + H2O → LiOH + H2 ↑
ii) Interstitial hydrides : These are the hydrides formed by the reaction of hydrogen with d-block and f-block elements. These are also called as metallic hydrides.
Eg : CrH, CrH2, ZnH2, ThH2
- The hydrogen of the metallic hydride occupy the intersticies of metallic lattice. Hence these are called as Interstitial hydrides.
- These are non-stiochiometric compounds
Eg : TiH1.5-1.8 LaH2.87 etc- - In these hydrides law of constant composition does not found.
- Metals of group 7, 8 and 9 do not form hydrides and in group 6 only chromium forms hydrides.
- The conductivity of these hydrides is less than the parent metals.
- The formation of metallic hydrides and their capacity to release hydrogen at high temperature are utilised in the purification of H2.
- Some metals can accomodate a very large volume of hydrogen and acts as storage media.
Question 5.
Explain any four of the chemical properties of water.
Answer:
i) Hydrolysis Reaction : The chemical interaction of a compound with water is called as hydrolysis. -» Water has high hydrating ability because of high dielectric constant.
Covalent as well as ionic compounds undergo hydrolysis.
Eg: P4O10(s) + 6H2O(l) → 4H3PO4(aq) (orthophosphoric acid)
NCl3 + 3H2O → NH3 + 3HOCl
SiCl4 + 2H2O → SiO2 + 4HCl
ii) Formation of hydrogen : Water can be reduced to dihydrogen by reacting with highly elec-tropositive metals.
2Ma(s) + 2H2O(l) → 2NaOH(aq) + H2(g)
Water is an important source of dihydrogen.
iii) Photosynthesis reaction : During photo synthesis reaction water gets oxidised to O2.
6CO2(g) + 6H2O(l) → C6H12O6(aq) + 6O2(g)
iv) Hydrates formation : By the association of water molecules of different types hydrated salts formed from the crystallisation of salts.
Eg : BaCl2.2H2O, CuSO4. 5H2O
v) Amphoteric Nature : Water has the ability to act as an acid as well as base. It is an amphoteric substance.
Eg: i) H2O(l) + NH3(aq) ⇌ OH–(aq) + NH4+(aq)
In this reaction H2O acts as Bronsted acid.
ii) H2O(l) + H2S(aq) ⇌ H3O+(aq) + HS–(aq)
In this reaction H2O acts as Bronsted base.
![]()
Question 6.
Explain the terms hard water and soft water. Write a note on the
i) ion-exchange method and
ii) calgon method for the removal of hardness of water.
Answer:
Hard water : Water does not give lather readily with soap is called hard water.
Hard water contains hardness. This hardness is due to presence of Ca, Mg soluble salts.
Presence of Ca, Mg – bicarbonates causes temporary hardness.
Presence of Ca, Mg – chlorides, sulphates causes permanent hardness.
Soft water: Water which give lather immediately with soap is called soft water.
(or)
Water which is free from soluble salts of Ca, Mg is called soft water.
i) Ion – Exchange method :
This method is useful to remove the permanent hardness of water.
- This method is also named as permutil (or) zeolite process.
- Permutit is the artificial zeolite, j.e sodium aluminium orthosilicate. (Na2Al2Si2O8xH2O (or) NaAlSiO4)
- Permutit is written in short form as Naz.
- When permutit is added to hard water, the following ion-exchange reactions takes place.
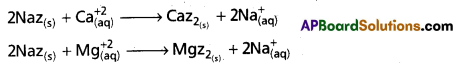
- Caz2 and Mgz2 are called as exhausted permutit. These are regenerated to permutit by the treatment with brine solution.
\(\mathrm{Caz}_{2_{(5)}}+2 \mathrm{Na}_{(\mathrm{aq})}^{+} \longrightarrow 2 \mathrm{Naz}_{(\mathrm{s})}+\mathrm{Ca}_{(\mathrm{aq})}^{+2}\)
ii) Calgon process : [A.P. Mar. 16]
- Calgon is sodium hexametaphosphate. [Na6P6O18 (or) (NaPO3)6]
- Calgon does not precipitate the Ca (or) Mg – salts but removes Ca+2 and Mg+2 ions from water. -4 The removal of Ca+2 (or) Mg+2 ions from water may takes place either by adsorption (or) by complex formation.
- Reactions :
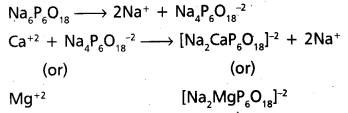
Question 7.
Write the chemical reaction to justify that hydrogen peroxide can function as on oxidizing as well as reducing agent. [T.S. Mar. 16]
Answer:
- H2O2 has the ability to function as an oxidising agent as well as reducing agent in both acid and alkaline solutions.
- In H2O2 oxidation state of oxygen is -1. It oxidised to O2. Here H2O2 is reductant.
- H2O2 can be reduced to H2O (or) OH–. Here H2O2 is oxidant.
- Oxidising action in acidic medium :
\(2 \mathrm{Fe}_{(a q)}^{+2}+2 \mathrm{H}_{(\mathrm{aq})}^{+}+\mathrm{H}_2 \mathrm{O}_{2_{(\text {aq })}} \longrightarrow 2 \mathrm{Fe}_{(\text {aq })}^{+3}+2 \mathrm{H}_2 \mathrm{O}_{(l)}\) - Reducing action in acidic medium :
\(2 \mathrm{MnO}_4^{-}+6 \mathrm{H}^{+}+5 \mathrm{H}_2 \mathrm{O}_2 \longrightarrow 2 \mathrm{Mn}^{+2}+8 \mathrm{H}_2 \mathrm{O}+5 \mathrm{O}_2\) - Oxidising action in basic medium :
2Fe+2 + H2O2 → 2Fe+3 + 2OH– - Reducing action in basic medium :
\(2 \mathrm{MnO}_4^{-}+3 \mathrm{H}_2 \mathrm{O}_2 \longrightarrow 2 \mathrm{MnO}_2+3 \mathrm{O}_2+2 \mathrm{H}_2 \mathrm{O}+2 \mathrm{OH}^{-}\)
![]()
Question 8.
Complete and balance the following chemical reactions :
i) PbS(s) + H2O2 →
ii) \(\mathrm{MnO}_{4_{(a q)}}^{-}\) + H2O2(aq) →
iii) CaO(s) + H2O(g) →
(iv) Ca3N2(s) + H2O(l) →
Classify the above into
a) hydrolysis
b) redox and
c) hydration reactions.
Answer:
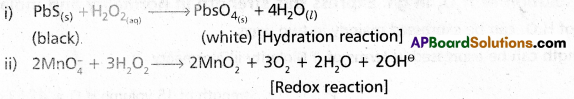
iii) CaO(s) + H2O(g) → Ca(OH)2
(iv) Ca3N2(s) + H2O(l) → 3Ca(OH)2 + 2NH3
(iii) and (iv) reactions are hydrolysis reactions
Question 9.
Discuss, with relevant chemical equations, various methods of preparing hydrogen peroxide. Which of these methods is useful to prepare D2O2 ?
Answer:
Preparations of H2O2:
i) From Acidified BaO2 removing the excess of water by evaporation under reduced pressure gives H2O2.
BaO2 . 8H2O(s) + H2SO4(aq) → BaSO4(s) + H2O2(aq) + 8H2O(l)
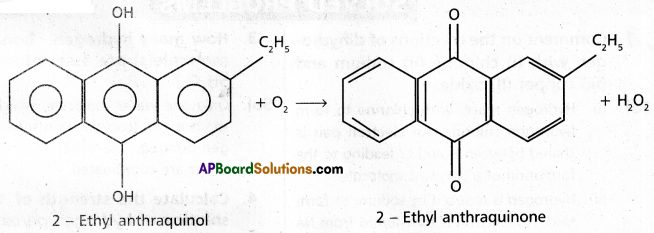
ii) Auto oxidation method : H2O2 is prepared industrially by the auto oxidation of 2-ethyl anthraquinol.
iii) Electrolysis of peroxo disulphuric acid : H2O2 is obtained by the electrolysis of 50% H2SO4 solution, peroxo disulphuric acid obtained undergo hydrolysis to form H2O2.
![]()
Preparation of D2O2: D2O2 is obtained by the reaction of K2S2O8 with heavy water. This is similar to above method (iii).
K2S2O8(s) + 2D2O(l) → 2KDSO4(aq) + D2O2(l)
![]()
Question 10.
In how many ways can you express the strength of H2O2 ? Calculate the strength of 15 volume solution of H2O2 in g/l. Express this strength in normality and molarity.
Answer:
Strength of H2O2 can be expressed majorly in two ways.
H2O2 strength can be expressed in terms of i) Molarity ii) Normality.
Problem :
Solution:
15 volume H2O2 solution means 1 lit, of H2O2
will give 15 lit, of O2 at STP
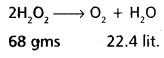
22.4 lit, of 02 produced from 68 gms of H2O2
15 lit, of 02 produced from x gms of H2O2
x = \(\frac{68 \times 15}{22.4}\) = 45.53 gms. of H2O2
∴ Strength of 15 volume H2O2 = 45.53 gms/lit.
= 4.5% H2O2
→ Molarity of the 15 volume H2O2 solution
= \(\frac{\mathrm{Wt}}{\mathrm{GMW} \times 1 \text { lit. }}\)
= \(\frac{45.53}{34 \times 1}\) = 1.339M.
→ Normality of the 15 volume H2O2 solution
= \(\frac{W t}{\text { G.E.W } \times 1 \text { lit. }}=\frac{45.53}{17 \times 1}\) = 2.678 N.
Solved Problems
Question 1.
Comment on the reactions of dihydro-gen with
- chlorine
- sodium and
- copper (II) oxide.
Solution:
- Hydrogen reacts with chlorine to form hydrogen chloride. An electron pair is shared between H and Cl leading to the formation of a covalent molecule.
- Hydrogen is reduced by sodium to form NaH. An electron is transferred from Na to H leading to the formation of an ionic compound, Na+ H .
- Hydrogen reduces copper(ll) oxide to copper and itself gets oxidised to H2O.
Question 2.
H2O has a higher boiling point than that of H2S. Give reasons.
Solution:
On the basis of molecular mass of H2O. Its boiling point is expected to be lower than that of H2S. However, due to higher electronegativity of O, the magnitude of hydrogen bonding in H2O will be quite appreciable. Hence, the boiling point of H2O will be higher than that of H2S.
![]()
Question 3.
How many hydrogen – bonded water molecule(s) are associated in CuSO4. 5H2O ?
Solution:
Only one water molecule, which is outside the brackets (coordination sphere), is hydrogen-bonded. The other four molecules of water are coordinated.
Question 4.
Calculate the strength of 10 volume solution of hydrogen peroxide.
Solution:
10 volume solution of H2O2 means that 1L of this H2O2 solution will give 10 L of oxygen at STP.
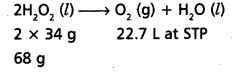
On the basis of above equation 22.4 L of O2 is produced from 68 g H2O2 at STP 10 L of O2 at STP is produced from \(\frac{68 \times 10}{22.4}\) g = 30.36g
≈ 30g H2O2
Therefore, strength of H2O2 in 10 volume H2O2 solution = 30.36 g/L = 3% H2O2 solution.