Andhra Pradesh BIEAP AP Inter 1st Year Chemistry Study Material 9th Lesson The s-Block Elements Textbook Questions and Answers.
AP Inter 1st Year Chemistry Study Material 9th Lesson The s-Block Elements
Very Short Answer Questions
Question 1.
Give reasons for the diagonal relationship observed in the periodic table.
Answer:
- Diagonal relationship is due to similar sizes of atoms (or) ions
- Diagonal relationship is due to similar electro negativities of the respective elements. Diagonally similar elements possess same polarising power.
- Polarizing Power = \(\frac{\text { ionic charge }}{\text { (ionic radius) }^2}\)
Question 2.
Write completly the electronic configurations of K and Rb.
Answer:
The electronic configuration of ‘K’ is 1s2 2s2 2p6 3s2 3p6 4s2
The electronic configuration of ‘Rb’ is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1.
![]()
Question 3.
Lithium salts are mostly hydrated. Why ?
Answer:
Hydration enthalpy of Li+ ion is very high. It has very high degree of hydration. So ‘Li’ salts are mostly hydrated.
Eg : LiCl . 2H2O.
Question 4.
Which of the alkali metals shows abnormal density ? What is the order of the varia-tion of density among the IA group elements.
Answer:
- ‘K’ has abnormal density among alkalimetals due to high inter atomic distances in crystal lattice.
- The order of the variation of density among the IA group elements as follows.
Li < Na > K < Rb < Cs.
Question 5.
Lithium reacts with water less vigorously than sodium. Give your reasons.
Answer:
Lithium reacts with water less vigorously than sodium.
Reasons :
- Lithium has small size.
- Lithium has very high hydration energy.
Question 6.
Lithium Iodide is the most covalent among the alkali metal halides. Give the reasons.
Answer:
Lithium iodide is the most covalent among the alkalimetal halides.
Reasons :
- The polarising capability of lithium ion is high.
- Li+ ion has very small size.
- Li+ ion has high tendency to distort electron cloud around the iodide ion.
![]()
Question 7.
In what respects lithium hydrogen carbonate differs from other alkali metal hydrogen carbonates.
Answer:
Lithium hydrogen carbonate cannot exist in solid form but remaining alkali metal hydrogen carbonates exist as solids.
Question 8.
Write the complete electronic configurations of any two alkaline earth metals.
Answer:
- The electronic configuration of ‘Mg’ is 1s2 2s2 2p6 3s2
- The electronic configuration of ‘Ca’ is 1s2 2s2 2p6 3s2 3p6 4s2.
Question 9.
Tell about the variation of m.pts., and b.pts among the alkaline earth metals.
Answer:
- The m.pts and b.pts of alkaline earth metals are higher than corresponding alkali metals due to smaller sizes.
- Due to low I.P. values the variation of m.pts and b.pts among alkaline earth metals is not sys-tematic.
Question 10.
What are the characterstic colours imparted by the HA elements ?
Answer:
Elements – Imparted colours towards flame
Calcium – Brick red
Strontium – Crimson red
Barium – Apple green
Beryllium – No colour
Magnesium – No colour
Question 11.
What happens when magnesium metal is burnt in air ?
Answer:
Magnesium metal burns with dazzling brilliance in air to give MgO and Mg3N2.
2 Mg + O2 → 2 MgO
3 Mg + N2 → Mg3N2.
![]()
Question 12.
Lithium carbonate is not so stable to heat as the other alkali metal carbonates. Explain.
Answer:
Lithium carbonate is not so stable to heat because Lithium has very small size and it polarises the large CO3-2 ion which leads to the formation of more stable Li2O and CO2.
As the electro positive character increases down the group, the stability of carbonats increase.
Question 13.
Write a balanced equation for the formation of ammoniated IIA metal ions from the metals in liquid ammonia.
Answer:
Alkaline earth metals dissolve in liquid ammonia to give deep blue black solutions forming ammoniated ions.
M + (x + y) NH3 → [M(NH3)x]2+ + 2 [e(NH3)y]–
From the above solutions, ammoniates [M(NH3)x]2+ can be recovered.
Question 14.
The fluorides of alkaline earth metals are relatively less soluble than their respective chlorides in water. Why ?
Answer:
Because of their high lattice energies fluorides of alkaline earth metals are relatively less soluble than their respective chlorides in water.
Question 15.
What happens when hydrated Mg (NO3)2 is heated ? Give the balanced equation.
Answer:
When hydrated Mg(NO3)2 is heated, it first loses the six water molecules and on further heating
decomposes to give the oxide.
2 Mg (NO3)2 → 2 MgO + 4NO2 + O2.
![]()
Question 16.
Why does the solubility of alkaline earth metal hydroxides in water increases down the group ?
Answer:
Among alkaline earth metal hydroxides, the anion being common the cationic radius will influence the lattice enthalpy. Since lattice enthalpy decreases much more than the hydration enthalpy with increasing ionic size, the solubility increases as we go down the group.
Question 17.
Why does the solubility of alkaline earth metal Carbonates and sulphates in water decrease down the group ?
Answer:
The size of anions being much larger compared to cations, the lattice enthalpy will remain almost constant within a particular group. Since the hydration enthalpies decrease down the group, solubility will decrease as found for alkaline earth metal carbonates and sulphates.
Question 18.
Write the average composition of Portland cement.
Answer:
Composition of port land cement is
Cao – 50 – 60%
Sio2 – 20 – 25%
Al2O3 – 5 – 10%
Mgo – 2 – 3%
Fe2O3 – 1 – 2% and
SO2 – 1 – 2%
Question 19.
Why is gypsum added to cement ?
Answer:
Gypsum is added to cement to slow down the process of setting of the cement and to get sufficiently hardened cement.
Question 20.
Why are alkali metals not found in the free state in nature ? [Mar. 13]
Answer:
Alkali metals are not found in the free state in nature because they readily lose their valency electron to form M+ ion (a nonvalent ion).
![]()
Question 21.
Potassium carbonate cannot be prepared by Solvay process. Why ?
Answer:
Potassium carbonate cannot be prepared by solvay process because potassium bi carbonate is more soluble and to be precipitated by the addition of ammonium bi carbonate to a saturated solution of potassium chloride.
Question 22.
Describe the important uses of caustic soda.
Answer:
Uses:
- It is used in petrol refining
- It is used in the purification of bauxite.
- It is used in manufacturing of soap, paper.
- It is used in manufacturing of antificial silk.
- It is used in manufacturing of so many chemically.
- It is used in textile industries for mercerising cotton fabrics.
- It is used in preparation of pure fats and oils.
- It is used in as laboratory reagent.
Question 23.
Describe the important uses of sodium carbonate.
Answer:
Uses:
- Na2CO3 is used in the manufacturing of glass.
- Na2CO3 is used in the manufactuing of borax, caustic soda.
- Na2CO3 is used in paper, paints and textile industries.
- Na2CO3 is used in softening of water.
- Na2CO3 is used in laundries.
- Na2CO3 is used an important laboratory reagent both in qualitative and quantitative analysis.
![]()
Question 24.
Describe the important uses of quick line.
Answer:
Uses:
- Quick lime is used in the purification of sugar.
- Quick lime is used in the manufacture of dyestuffs.
- Quick lime is used in the manufacture of Na2CO3 from NaOH.
- It is an important material for manufacturing of cement and it is the cheapest form of alkali.
Question 25.
Draw the structures of
- BeCl2 (vapour) and
- BeCl2 (Solid).
Answer:
- In vapour phase BeCl2 forms a bridged dimer which disociates into monomer at high temperatures (around 1200 k)
Cl – Be – Cl - In solid state BeCl2 has a chain structure.
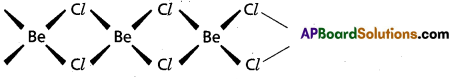
Question 26.
Describe the importance of Plaster of Paris.
Answer:
- Plaster of paris has an important property of setting with water.
- It forms a hard solid in 5 to 15 min. When it is mixed with suitable quantity of water.
- It is majorly used in building industry and as well as plasters.
- It is used in the bone fractures (or) sprain conditions.
- It is used in dentistry.
- It is used in manufacturing status and busts.
Question 27.
Which of the alkaline earth metal carbonates is thermally the most stable ? Why ?
Answer:
Among Alkaline earth metal carbonates BaCO3 is thermally most stable.
Reason :
As the cationic size increases thermal stability also increases. So BaCO3 is most stable thermally.
![]()
Question 28.
Write balanced equations for the reactions between
- Na2O2 and water
- K2O and water.
Answer:
- Na2O2 + 2H2O → 2 NaOH + H2O2
- K2O + H2O → 2 KOH.
Short Answer Questions
Question 1.
Alkali metals and their salts impart characteristic colours to an oxidizing flame. Explain the resonizing flame. Explain the reason.
Answer:
Alkali metals and their salts impart characterstic colours to an oxidizing flame.
Reasons :
The heat from the flame excites the outer most orbital electron to a higher energy level. When the excited electron emitts the radiation and comes back to the ground state. This falls in the visible region.
Question 2.
What makes caesium and potassium useful as electrodes in photoelectric cells ?
Answer:
- Alkali metals can be detected by the respective flame tests and can be determined by the flame photo metry (or) atomic absorption spectroscopy.
- Alkali metals when irradiated with light, the light energy absorbed may be sufficient to make an atom lose electron.
- This makes caesium and potassium useful as electrodes in photo electric cells.
Question 3.
Write a short note on the reactivity of alkali metals towards air.
Answer:
- The alkali metals forms their oxides in presence of dry air and tarnished.
- These oxides reacts with moisture to form hydroxides.
- They burn vigorously in oxygen and forms oxides.
- Lithium forms Lithium monoxide.
- Sodium forms monoxide with limited supply of oxygen and peroxide with excess of oxygen.
- Other metals of this group forms super oxides. The super oxide ion (O2–) is stable only in presence of large cations.
Reactions :
4Li + O2 → 2 Li2O
4Na + O2 (Limited) → 2 Na2O2
2Na + O2 (Excess) → Na2O2
K + O2 (Excess) → KO2
Lithium shows a different character. It reacts directly with nitrogen of air and forms Li3N (Lithium nitride).
![]()
Question 4.
Give any two uses for each of the following metals.
- Lithium
- Sodium.
Answer:
- Uses of Lithium : –
- ‘Li’ metal is used to make alloys.
Eg: 1) Lithium with lead forms an alloy which is used for making white metal bearings for motor engines.
2) Lithium with aluminium forms alloys which are used to make air craft parts. - ‘Li’ metal is used in thermo nuclear reactions.
- ‘Li’ metal is used to make electro chemical cells.
2) Uses of sodium : –
- Sodium metal is used to make Na and Pb alloy needed to make TEL. this TEL (tetra ethyl lead) is used as antiknock additives to petrol.
- Liquid ‘Na’ metal is used as a coolant in fast breeder nuclear reactors.
- ‘Na’ metal is used in the manufacturing of rabber.
Question 5.
Give an account of properties of washing soda.
Answer:
Properties of washing soda : –
- Na2CO3 is a white crystalline solid.
- Na2CO3 exists as a decahydrate Na2CO3. 10H2O which is called washing soda.
- Na2CO3 is readily soluble in water.
- Na2CO3 (deca hydrate) when heated it loses the water molecules and forms monohydrate. This monohydrate on heating above 373 K it forms anhydrous form which is called soda ash, a white powder.
Reactions : –
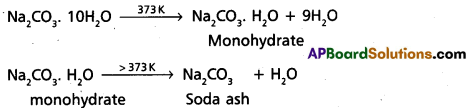
- Na2CO3 (aq) solution is alkaline (basic) in nature Because it under goes anionic hydrolysis. (PH > 7).
CO3-2 + H2O → HCO3– + OH–
Question 6.
Mention some uses of sodium carbonate.
Answer:
Uses :
- Na2CO3 is used in the manufacturing of glass.
- Na2CO3 is used in the manufacturing of borax, caustic soda.
- Na2CO3 is used in paper, paints and textile industries. .
- Na2CO3 is used in softening of water.
- Na2CO3 is used in laundries.
- Na2CO3 is used an important laboratory reagent both in qualitative and quantitative analysis.
![]()
Question 7.
How do you obtain pure sodium chloride from a crude sample ?
Answer:
- Crude sodium chloride is obtained by the crystallisation of brine solution.
- Crude sodium chloride contains sodium sulphate, calcium sulphate, calcium chloride and magnesium chloride.
- CaCl2 and MgCl2 are the impurities in the Crude NaCl because these absorb moisture easily from the atmosphere.
- Pure sodium chloride’s obtained by dissolving Crude NaCl in minimum amount of water and filtered to remove insoluble impurities.
- This solution is saturated with HCl gas. Then crystals of pure NaCl are separated out.
- Ca and Mg chloride are more soluble in solution than NaCl and these remained in the solution.
Question 8.
What do you know about Castner – Kellner process ? Write the principle involved in it.
Answer:
- Castner – Kellner process is a commercial method used for the preparation of sodium hydroxide.
- In this process sodium hydroxide is prepared by the electrolysis of sodium chloride in Castner – Kellner cell.
- Brine solution is electrolysed using a mercury cathode and a carbon anode.
- Sodium metal is formed at cathode and it combine with mercury to form sodium amalgam. Chlorine gas is evolved at anode.
- The amalgam is treated with water to form, sodium hydroxide.
Cell Reactions :
2NaCl → 2Na+ + 2Cl–
2Na+ + 2e– 2Na – amalgam
2Na – amalgam
2Cl– → Cl2 + 2e–
2Na – amalgam + 2H2O → 2NaOH + 2Hg + H2 - This process is also called as mercury cathode process.
Question 9.
Write a few applications of caustic soda.
Answer:
Uses :
- It is used in petrol refining
- It is used in the purification of bauxite.
- It is used in manufacturing of soap, paper.
- It is used in manufacturing of antificial silk.
- It is used in manufacturing of so many chemically.
- It is used in textile industries for mercerising cotton fabrics.
- It is used in preparation of pure fats and oils.
- It is used in as laboratory reagent.
![]()
Question 10.
Give an account of the biological importance of Na+ and K+ ions.
Answer:
- Na+ ions participate in the transmission of nerve signals.
- Na+ ions regulates the flow of water accross cell membranes.
- Na+ ions responsible for transport of sugars and amino acids into cells.
- K+ ions are useful in activating enzymes.
- K+ ions participate in the oxidation of glucose to produce ATP.
- K+ along with Na+ responsible for the transmission of nerve signals.
Question 11.
Mention the important uses of Mg metal.
Answer:
- Magnesium forms so many useful alloys with Al, Zn, Mn and Sn.
- Mg – Al alloys are useful in air – craft construction.
- Mg powder and ribbon is used in flash powders bulbs.
- Mg is used in incendiary bombs and signals.
- Milk of magnesice is used as antacid in medicine.
- MgCO3 is the main ingradient in tooth pastes.
Question 12.
Show that Be(OH)2 is amphoteric in nature.
Answer:
- Be(OH)2 is amphoteric in nature. This can be evidented by the following reactions.
- Be(OH)2 reacts with both acids and alkalis.
Be(OH)2 + 2OH– [Be(OH)4]2- (Beryllation)
Be(OH)2 + 2HCl + 2H2O → [Be(OH)4]Cl2 - Hence Be(OH)2 is amphoteric in nature.
![]()
Question 13.
Write a note on anomalous behaviour of beryllium.
Answer:
Anomalous characters of Be:
As was already discussed in the earlier sections, the first element shows some differences from the properties of the other elements in the group. Be differs from the other alkaline earth metals because of its small size and high electronegativity. Be differs from the other elements in the following aspects.
- Be compounds are predominantly covalent due to its high polarizing power and its salts are readily Hydrolyzed.
- Be is not easily affected by dry air and does not decompose water at ordinary temperature.
- Be is an amphoteric metal. It dissolves in alkali solutions forming beryllates.
- Be SO4 is soluble in water whereas the sulphates of Ca, Sr and Ba are not soluble.
- Be and its salts do not respond to flame test while Ca, Sr and Ba give characteristic flame colours.
- Be forms many complexes while the heavier elements do not show a great tendency to form complexes.
- Be has a maximum covalency of 4 while other can have a maximum covalency of 6.
Question 14.
Be shows diagonal relationship with Al. Discuss.
Answer:
- ‘Be’ shows diagonal relation ship with ‘Al’.
- The ionic radius of Be+2 is nearly same as that of Al+3 so ‘Be’ resembles ‘Al’ in several ways.
- Al, Be both not readily reacts with acids. This is due to the presence of an oxide film on the surface of metal.
- Al(OH)3, Be(OH)2 both similarly dissolves in excess of alkali and forms Beryllate ion [Be(OH)3]2- and Aluminate [Al(OH)4] ions respectively.
- The chlorides of Be, Al have bridged chloride structures in vapour phase.
- Both the chlorides of Be, Al used as strong Lewis acids.
- Both the chlorides of Be, Al used in Friedal craft catalysts.
- Be, Al ions have strong tendency to form complexes.
Question 15.
What is Plaster of Paris ? Write a short note on it. [T.S. Mar. 16]
Answer:
Plaster of paris is the hemi hydrate of CaSO4 with formula CaSO4. \(\frac{1}{2}\)H2O.
Preparation: –
Plaster of paris is obtained by heating gypsum at 393 K.
![]()
- If temperature is used greater than 393 K then an hydrous CaSO4 is formed which is called ‘dead burnt plaster’.
- Plaster of paris has an important property of setting with water.
- It forms a hard solid in 5 to 15 min. when it is mixed with suitable quantity of water.
- It is majorly used in building industry and as well as plasters.
- It is used in the bone fractures (or) sprain conditions.
- It is used in dentistry.
- It is used in manufacturing status and busts.
![]()
Question 16.
In what ways lithium shows similarities to magnesium in its chemical behaviour ?
Answer:
Diagonal relationship of Li : In the periodic table an element of a group in the 2nd period shows similar properties with the second element of the next group in the third period. This relation is known as diagonal relationship. For examples. Lithium and Magnesium show diagonal relationship. The elements show that diagonal relationship have similar polarizing powers, electronegativities, nature of the compounds. The diagonal similarity may be due to the effects of size and charge. For example, charge per unit area.
Lithium shows similarity to Magnesium in the following respects.
a) Lithium is slow to react with water. Magnesium decomposes water only in the hot condition.
2Li + 2H2O → + H2;
Mg + 2H2O → Mg(OH)2 + H2
b) Lithium combines directly with N2 forming nitride.
6Li + N2 → 2Li3N
c) Both Lithium and Magnesium give only monoxides Li2O, MgO.
d) Lithium chloride is deliquescent like MgCl2, LiCl undergoes hydrolysis to a smaller extent in hot water in a similar way to MgCl2.
e) Due to their covalent nature, the halides Lithium and Magnesium are soluble in organic solvents.
f) Both Li+ and Mg+2 are highly hydrated.
g) The Carbonates, Phosphates and Fluorides of both Li and Mg are sparingly soluble in water.
h) Lithium alkyls (Li+ R–) are chemically similar to Grignard reagents in organic synthesis.
Question 17.
When an alkali metal dissolves in liquid ammonia the solution can acquire different colours. Explain the reasons for this type of colour change.
Answer:
- The alkali metals dissolve in liquid NH3 and gives deep blue solutions. These are conducting in nature.
- The blue colour of the solution is due to the ammoniated electrons which absorbs energy in the visible region of light and thus imparts blue colour to the solution.
- These solutions are paramagnetic and on standing liberate hydrogen resulting in the formation of amide.
M + (x + y) NH3 → [M(NH3)x]+ + [e(NH3)y]–
M(am)+ + e– + NH3 → MNH2(am) + 1/2 H2(g) - In concentrated solution the blue colour Changes to bronze.colour on warming and becomes did magnetic.
Question 18.
What happens when
- Sodium metal is dropped in water ?
- Sodium metal is heated in a free supply of air ?
- Sodium peroxide dissolves in water ?
Answer:
- Sodium metal when dropped in water it reacts with water vigourously and liberates H2 gas.
2Na + 2H2O → 2NaOH + H2 - Sodium metal is heated in free supply of air to form sodium peroxide.
2Na + O2 → Na2O2 (sodium peroxide) - Sodium peroxide dissolves in water and forms NaOH and hydrogen peroxide.
Na2O2 + 2H2O → 2NaOH + H2O2
![]()
Question 19.
States as to why
i) An aqueous solution of Na2CO3– is alkaline ;
ii) Alkali metals are prepared by the electrolysis of their fused chlorides ?
Answer:
i) An aqueous solution of Na2CO3 is alkaline. This is due to anionic (CO3-2) hydrolysis.
CO3-2 + H2O → HCO3-2 + OH–
∴ PH > 7. So the solution is alkaline in nature.
ii) Chemically alkali metals are highly reactive and they are placed at top in the electro chemical series.
∴ Common methods of extraction of the metals are not applicable for the alkali metals. So electrolytic reduction of their used chlorides is the possible method for extracting alkali metals.
Eg: ‘Na’ metal obtained from fused ‘NaCl’.
Question 20.
How would you explain the following observations ?
- BeO is almost insoluble but BeSO4 is soluble in water ?
- BaO is soluble but BaSO4 is insoluble in water ?
Answer:
- BeO has amphoteric nature and the solubility in water is low because of its covalent nature.
BeSO4 is soluble in water. This is due to greater hydration energy of Be+2 ion. - BaO is soluble in water because of its ionic nature.
BaSO4 is insoluble in water because of low hydration energy of Ba+2 ion.
Long Answer Questions
Question 1.
Justify the inclusion of alkali metals in the same group of the periodic table with reference to the following :
i) Electronic configuration
ii) Reducing nature
iii) Oxides and hydroxides.
Answer:
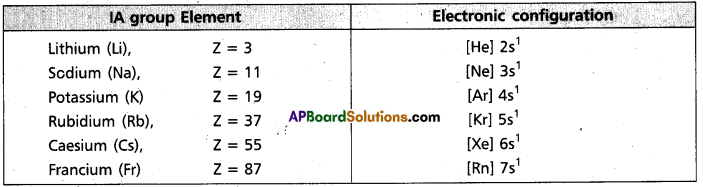
i) Electronic configuration : All the alkali metals have one valence electron, ns1.
ii) Reducing nature :
- Alkali metals are strong reducing agents.
- ‘Li’ is most powerful reducing agent and ‘Na’ is poor reducing agent. .
The standard electrode potential (E°) is the measure of reducing power. - ‘Li’ has highest hydration enthalpy. It has high negative S.E.P. (E°) hence it has high reducing power.
iii) Oxides and hydroxides : –
- The alkali metals forms their oxides in presence of dry air and tarnished.
- These oxides reacts with , .noisture to form hydroxides.
- They burn vigorously in oxygen and forms oxides.
- Lithium forms lithium monoxide.
- Sodium forms monoxide with limited supply of oxygen and peroxide with’ excess of oxygen
- Other metals of this group forms super oxides. The super oxide ion (O2–) is stable only in presence of large cations.
Reactions:
4Li + O2 → 2 Li2O
4Na + O2 (Limited) → 2 Na2O
2 Na + O2 (Excess) → Na2O2
K + O2 (Excess) → KO2
Lithium shows a different character. It reacts directly with nitrogen of air and forms Li3N (Lithium nitride).
- Alkali metal oxides easily hydrolysed by water to form the hydroxides.
Monoxide : M2O + H2O → 2MOH
Peroxide : M2O2 + 2H2O → 2MOH + H2O2
Superoxide : 2MO2 + 2H2O → 2MOH + H2O2 + O2 - Oxides, peroxides are colourless whereas superoxides are coloured because of their para mag-netic property.
- Hydroxides are white crystalline solids.
- Hydroxides are strong bases and dissolved freely in water and evolve much heat.
![]()
Question 2.
Write an essay on the differences between lithium and other alkali metals.
Answer:
Anomalous properties of Lithium : In the periodic table some representative elements of different series show similarities, known as diagonal relationship. Li, of the alkali metals, show such a similarity with Mg of II group. That means it differs from alkali metals. Some of the important abnormal characters of lithium are given below.
a) Lithium is hard metal while other alkali metals are soft and can be cut with the knife. Its melting point and boiling point are high.
b) Lithium directly unites with N2 while no other alkali metal combines directly.
6Li + N2 → 2Li3N
c) Lithium element forms a carbide on direct combination. Group IA elements do not form directly. But all these elements are known to give carbides.
d) The solubilities of Lithium Hydroxide (LiOH), Lithium Carbonate (Li2CO3), Lithium Phosphate (Li3PO4) and Lithium Fluoride (LiF), are very less compared to the high solubilities of the other alkali metal compounds.
e) Lithium Hydroxide is a weaker alkali than the alkali metal Hydroxides. Basic nature of other alkali metal Hydroxides is more than Li(OH). Because of this Lithium Hydroxide Carbonate, nitrates are thermally unstable.
Question 3.
Discuss the preparation and properties of sodium carbonate.
Answer:
Preparation :
- Sodium carbonate is prepared by solvay process.
- In this process sodium chloride reacts with ammonium bicarbonate and gets precipitated the low soluble sodium bicarbonate.
- The lather is prepared by passing CO2 into a concentrated solution of NaCl saturated with ammonia. Here (NH4)2CO3 followed by NH4 HCO3 are formed.
Chemical equations involved :
2NH3 + H2O + CO2 → (NH4)2 CO3
(NH4)2 CO3 + H2O + CO2 → 2NH4HCO3
NH4HCO3 + NaCl → NH4Cl + NaHCO3 - The separated NaHC03 Crystals heated to get Na2CO3.
2NaHCO3 → Na2CO3 + H2O + CO2 - In this process ammonia is regenerated by Ca(OH)2.
2NH4Cl + Ca(OH)2 → 2NH3 + CaCl2 + H2O
Properties of washing soda :
- Na2CO3 is a white crystalline solid.
- Na2CO3 exists as a decahydrate Na2CO3. 10H2O which is called washing soda.
- Na2CO3 is readily soluble in water.
- Na2CO3 (decahydrate) when heated it loses the water molecules and forms monohydrate. This monohydrate on heating above 373 K it forms anhydrous form which is called soda ash, a white powder.
Reactions : –

- Na2CO3 (aq) solution is alkaline (basic) in nature Because it under goes anionic hydrolysis. (PH > 7).
CO3-2 + H2O → HCO3– + OH–
Uses :
- Na2CO3 is used in the manufactuing of glass.
- Na2CO3 is used in the manufactuing of borax, caustic soda.
- Na2CO3 is used in paper, paints and textile industries.
- Na2CO3 is used in softening of water.
- Na2CO3 is used in laundries.
- Na2CO3 is used an important laboratory reagent both in qualitative and quantitative analysis.
![]()
Question 4.
Discuss the similarities between alkaline earth metals and gradation in the following aspects.
i) Electronic configuration
ii) Hydration enthalpies
iii) Nature of oxides and hydroxides.
Answer:
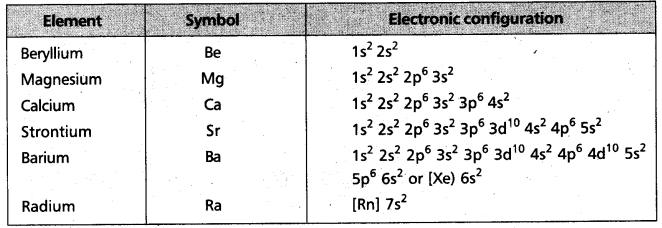
i) Electronic configuration :
The general electronic configuration of alkaline earth metals represented by [noble gas] ns2.
ii) Hydration Enthalpies :
- The hydration enthalpies of alkaline earth metal ions decrease with increase in ionic size down the group.
Be+2 > Mg+2 > Ca+2 > Sr+2 > Ba+2 - Hydration enthalpies of these elements ions are larger than those of alkali metal ions.
Eg : MgCl2 MgCl2 . 6H2O
CaCl2 CaCl2 . 6H2O
iii) Nature of oxides and hydroxides : –
- Alkaline earth metals forms oxides of type MO.
- These are formed by burning in oxygen.
- BeO is amphoteric and covalent in nature whereas other oxides are ionic and basic in nature. Other oxides i.e., except BeO forms hydroxides with water.
Eg : MgO + H2O → Mg(OH)2 - The solubility, thermal stability and the basic character of these hydroxides increase with increase of atomic no. from Mg(OH)2 to Ba(OH)2. H
- These hydroxides are less basic, less stable than alkali metal hydroxide.
- Be(OH)2 is amphoteric in nature. This can be evidented by the following reactions.
- Be(OH)2 reacts with both acids and alkalis.
Be(OH)2 + 2OH– [Be(OH)4]2- (Beryllation)
Be(OH)2 + 2HCl + 2H2O → [Be(OH)4]Cl2 - Hence Be(OH)2 is amphoteric in nature.
Question 5.
Discuss on;
i) Carbonates
ii) Sulphates and
iii) Nitrates of alkaline earth metals.
Answer:
i) Carbonates :
- Alkaline earth metals forms MCO3 type carbonates.
- These Carbonates are insoluble in water.
- The solubility of these carbonates in water decreases as the atomic no.of the element increases.
- The thermal stability increases with increasing cationic size.
- These carbonates decompose on heating to give CO2
CaCO3 Cao + CO2
Cao + CO2 - BeCO3 is unstable and kept only in at atmosphere of CO2.
ii) Sulphates :
- Alkaline earth metals forms MSO4 type sulphates.
- These are white solids and are stable to heat.
- BeSO4 and MgSO4 are readily soluble in water due to high hydration enthalies of Be2+, Mg2+.
- The solubility decrease from CaSO4 to BaSO4.
iii) Nitrates :
- Alkaline earth metals forms M(NO3)2 type Nitrates.
- These are formed by the reaction of carbonates in dil.HNO3.
- Mg(N03)2 crystallises with six water molecules and Ba(NO3)2 is an hydrous.
- All of these decompose on heating to give the respective oxides.
2M(NO3)2 → 2MO + 4NO2 + O2
M = Be, Mg, Ca, Sr, Ba.
![]()
Question 6.
What are the common physical and chemical features of alkali metals ?
Answer:
Physical features :
- Alkali metals are silvery white, soft and light metals.
- These elements have low density which increases down the group from Li to Cs. (one exception density of K < density of Na).
- The m.pts, b.pts of alkali metals are low.
- Alkali metals and their salts impart characterstic colours to an oxidizing flame.
Reasons :
The heat from the flame excities the outer most orbital electron to a higher energy level. When the excited electron emitts the radiation and comes back to the ground state. This falls in the visible region.
- Alkali metals can be detected by the respective flame tests and can be determined by the flame photo metry (or) atomic absorption spectroscopy.
- Alkali metals when irradiated with light, the light energy absorbed may be sufficient to make an atom lose electron. .
- This makes calsium and potassium useful as electrodes in photo electric cells.
Chemical features : –
i) Reactivity towards Air : —
- The alkali metals forms their oxides in presence of dry air and tarnished.
- These oxides reacts with moisture to form hydroxides.
- They burn vigorously in oxygen and forms oxides.
- Lithium forms Lithium monoxide.
- Sodium forms monoxide with limited supply of oxygen and peroxide with excess of oxygen.
- Other metals of this group forms super oxides. The super oxide ion (O2–) is stable only in presence of large cations.
- Reasons :
4Li + O2 → 2 Li2O
4Na + O2 (Limited) → 2 Na2O
2Na + O2 (Excess) → Na2O2
K + O2 (Excess) → KO2 - Lithium shows a different character. It reacts directly with nitrogen of air and forms Li3N (Lithium nitride).
ii) Reactivity with H2: Alkali metals react with H2 directly at 300 – 600° C and form hydrides. The reaction can be written as follows :
![]()
Where M = Li, Na, K, Rb or Cs. These hydrides are ionic in nature. Their ionic nature increases with the metalic nature of alkali metals.
iii) Reactivity with halogens : All the alkali’ metals react with halogens to give the binary compounds. The chemical reactivity in the alkali metals increases with increase in atomic number.
2M + X2 → 2MX (where M is any alkali metal)
All the metal halides are ionic compounds.
iv) Reactivity with water : The alkali metals decompose water vigorously and liberate hydrogen gas! The chemical reactivity of these metals increases as the atomic number increases. The metal hydroxides are formed.
2M + 2H2O → 2MOH + H2
Where M = any one of the alkali metals.
ii) Reducing nature :
- Alkali metals are strong reducing agents.
- ‘Li1 is most powerful reducing agent and ‘Na’ is poor reducing agent.
- The standard electrode potential (E°) is the measure of reducing power.
- ‘Li’ has highest hydration enthalpy. It has high negative S.E.P. (E°) hence it has high reducing power.
- The alkali metals dissolves in liquid NH3 and gives deep blue solutions. These are conducting in nature.
- The blue colour of the solution is due to the ammoniated electrons which absorbs energy in the visible region of light and thus imparts blue colour to the solution.
- These solutions are paramagnetic and on standing Liberate hydrogen resulting in the formation of amide.
M + (x + y) NH3 → [M(NH3)x]+ + [e(NH3)y]–
M(am)+ + e– + NH3 → MNH2(am) + 1/2 H2(g) - In concentrated solution the blue colour Changes to bronze.colour on warming and becomes did magnetic.
![]()
Question 7.
Discuss the general characterstics and gradation in properties of alkaline earth metals.
Answer:
The general characterstics and gradation in properties of alkaline earth metals follows.
i) Oxides and hydroxides :
- Alkaline earth metals forms oxides of type Mo.
- These are formed by burning in oxygen.
- BeO is amphoteric and covalent in nature where as other oxides are ionic and basic in nature.
- Other oxides i.e., except Beo forms hydroxides with water.
Eg : MgO + H2O → Mg(OH)2 - The solubility, thermal stability and the basic character of these hydroxides increase with increase of atomic no. from Mg(OH)2 to Ba(OH)2.
- These hydroxides are less basic, less stable than alkali metal hydroxide.
- Be(OH)2 is amphoteric in nature. This can be evidented by the following reactions.
- Be(OH)2 reacts with both acids and alkalis.
Be(OH)2 + 2OH– [Be(OH)4]2- (Beryllation)
Be(OH)2 + 2HCl + 2H2O → [Be(OH)4]Cl2 - Hence Be(OH)2 is amphoteric in nature.
ii) Halides:-
- These forms MX2 type halides.
- Except Be – halides, all other halides of these metals are ionic.
- Be – halides are covalent and soluble in organic solvents.
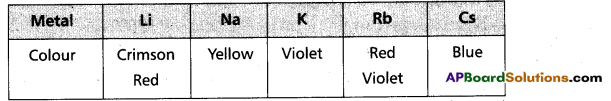
- In vapour phase BeCl2 forms a bridged dimer which disociates into monomer at high temperatures (around 1200 k)
Cl – Be – Cl - In solid state BeCl2 has a chain structure.
- The tendency to form halide hydrates gradually decreases – down the group.
Eg : MgCl2, 8H2O, CaCl2, 6H2O, BaCl2. 2H2O. - Ca, Sr and Ba halides, dehydration can be done by heating.
- Fluorides are less soluble than the chlorides due to their high lattice energies.
i) Carbonates :
- Alkaline earth metals forms MCO3 type carbonates.
- These Carbonates are insoluble in water.
- The solubility of these carbonates in water decreases as the atomic no.of the element increases.
- The thermal stability increases with increasing cationic size.
- These carbonates decompose on heating to give CO2
CaCO3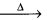 Cao + CO2
Cao + CO2 - BeCO3 is unstable and kept only in at atmosphere of CO2.
ii) Sulphates:
- Alkaline earth metals forms MSO4 type sulphates.
- These are white solids and are stable to heat.
- BeSO4 and MgSO4 are readily soluble in water due to high hydration enthalpies of Be2+, Mg2+.
- The solubility decrease from CaSO4 to BaSO4.
iii) Nitrates:
- Alkaline earth metals forms M(NO3)2 type Nitrates.
- These are formed by the reaction of carbonates in dil.HNO3.
- Mg(NO3)2 crystallises with six water molecules and Ba(NO3)2 is an hydrous.
- All of these decompose on heating to give the respective oxides.
2M(NO3)2 → 2MO + 4NO2 + O2
M = Be, Mg, Ca, Sr, Ba.
![]()
Question 8.
Discuss the various reactions that occur in the solvay process. [A.P. Mar. 16]
Answer:
Preparation:
- Sodium carbonate is prepared by solvay process.
- In this process sodium chloride reacts with ammonium bicarbonate and gets precipitated the low soluble sodium bicarbonate. ‘
- The lather is prepared by passing CO2 in to a concentrated solution of NaCl saturated with ammonia. Here (NH4)2CO3 followed by NH4 HCO3 are formed.
- Chemical equations involved:
2NH3 + H2O + CO2 → (NH4)2 CO3
(NH4)2 CO3 + H2O + CO2 → 2NH4HCO3
NH4HCO3 + NaCl → NH4Cl + NaHCO3 - The separated NaHCO3 Crystals heated to get Na2CO3.
2NaHCO3 → Na2CO3 + H2O + CO2 - In this process ammonia is regenerated by Ca(OH)2.
2NH4Cl + Ca(OH)2 → 2NH3 + CaCl2 + H2O
Question 9.
Starting with sodium chloride how would you proceed to prepare
i) Sodium metal
ii) Sodium hydroxide
iii) Sodium peroxide
iv) sodium carbonate.
Answer:
i) Fused NaCl on electrolysis gives sodium metal.
2NaCl → 2Na+ + 2Cl–
2Na+ + 2e– → 2Na (Cathode)
2Cl– → Cl2 + 2e– (anode)
ii)
- Castner-Kellner process is a commercial method used for the preparation of sodium hydroxide.
- In this process sodium hydroxide is prepared by the electrolysis of sodium chloride in Castner – Kellner cel..
- Brine solution is electrolysed using a mercury cathode and a carbon anode.
- Sodium metal is formed at cathode and it combine with mercury to form sodium amalgam. Chlorine gas is evolved at anode.
- The amalgam is streated with water to form sodium hydroxide.
Cell Reactions:
2NaCl → 2Na+ + 2Cl–
2Na+ + 2e–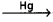 2Na – amalgam
2Na – amalgam
2Cl– → Cl2 + 2e–
2Na-amalgam + 2H2O → 2NaOH + 2Hg + H2 - This process is also called as mercury cathode process.
iii) The obtained Na – metal reacts with excess of oxygen to form sodium peroxide.
2Na + O2 → Na2O2 (Sodium peroxide.)
iv) Preparation:
- Sodium carbonate is prepared by solvay process.
- In this process sodium chloride reacts with ammonium bicarbonate and gets precipitated the low soluble sodium bicarbonate.
- The lather is prepared by passing CO2 into a concentrated solution of NaCl saturated with ammonia. Here (NH4)2CO3 followed by NH4 HCO3 are formed.
Question 10.
What happens when
i) Magnesium is burnt in air ?
ii) Quick lime is heated with silica
iii) Chlorine reacts with slaked lime
iv) calcium nitrate is strongly heated.
Answer:
i) Magnesium burns with dazzling brilliance in air to give MgO and Mg3N2.
2Mg + O2 →2MgO
3Mg + N2 → Mg3N2
ii) Quick lime heated with silica to form calcium silicate
Cao + SiO2 → CaSio3
iii) Slaked lime reacts with chlorine gas to form bleaching powder.
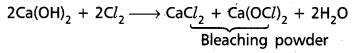
iv) Calcium nitrate on strong heating to form respective oxide
2Ca(NO3)2 → 2CaO + 4NO2 + O2.
![]()
Question 11.
Explain the significance of sodium, potassium, magnesium and calcium in biological fluids.
Answer:
Biological importance of Na, K.
- Na+ ions participate in the transmission of nerve signals.
- Na+ ions regulates the flow of water accross cell membranes.
- Na+ ions responsible for transport of sugars and amino acids into cells.
- K+ ions are useful in activating enzymes.
- K+ ions participate in the oxidation of glucose to produce ATP.
- K+ along with Na+ responsible for the transmission of nerve signals.
Biological importance of Mg and Ca :
Role of Mg2+ in biology :
- Mg2+ ions are concentrated in animal cells.
- Enzymes like “phosphohydrolases1 and ‘Phospho transferases’ contain Mg2+ ions. These enzymes participate in ATP reactions and release energy in the process. Mg2+ forms a complex with ATP.
- Mg2+ is a constituent of chlorophyll, the green component of plants.
Role of Ca+2:
About 99% of body calcium is present in bones and teeth. It also plays important roles in neuromuscular function, interneuronal transmission, cell membrance integrity and blood coaqulation.
The calcium concentration in plasma is regulated at about 100 mg/Lit. It is maintained by two hormones, calcitonin and parathyroid hormone. Ca2+ ion are necessary for muscle contraction.
![]()
Question 12.
Write few lines about cement ?
Answer:
- Cement is an important building material.
- It is also called portland cement.
- Cement is obtained by combining a material rich in lime, CaO with other material such as clay which contains Sio2 along with the oxides of Al, Fe and Mg.
- Composition of port land cement is
Cao – 50 – 60%
Sio2 – 20 – 25%
Al2O3 – 5 – 10%
Mgo – 2 – 3%
Fe2O3 – 1 – 2%
and SO2 – 1 – 2% - For a good quality of cement the ratio of SiO2 to Al2O3 is between 2.5 and 4 and the ratio of lime (Co) to the total of the oxides of SiO2, Al2O3 and Fe2O3 is as close as ‘2’.
- The raw materials used for the manufacture of cement are lime stone and clay.
Clay + lime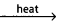 cement clinker.
cement clinker. - This cement clinker mixed with 2 – 3% by wt. of gypsum to form cement.
- Important ingradients in portland cement are
Ca2SiO4 – 26%, Ca3SiO5 – 51 % and Ca3Al2O6 – 11 %
Setting of Cement: –
- Cement mixed with water to give a hard mass i.e setting of cement takes place.
- This is due to the hydration of molecules of the cement.
- The purpose of adding gypsum is to slow down the process of setting and to get sufficiently hardness.
Uses:
- It is used in concrete and rein forced concrete.
- It is used in plastering.
- It is used in construction of bridges, dams and buildings.
Solved Problems
Question 1.
What is the oxidation state of K in KO2 ?
Solution:
The superoxide species is represented as O2–; since the compound is neutral, the oxidation state of potassium is +1.
Question 2.
The EΘ for Cl2 / Cl– is + 1.36, for I2/I– is + 0.53, for Ag+/Ag is + 0.79, Na+/Na is – 2.71 and for Li+/Li is – 3.04. Arrange the following ionic species in decreasing order of reducing strength : I–, Ag, Cl–, Li, Na
Solution:
The order is Li > Na > I– > Ag > Cl–.
![]()
Question 3.
Why is KO2 paramagnetic ? [T.S. Mar. 16]
Solution:
The superoxide O2– is paramagnetic because of one unpaired electron in π*2p molecular orbital.
Question 4.
Why does the solubility of alkaline earth metal hydroxides in water increases down the group ?
Solution:
Among alkaline earth metal hydroxides, the anion being common the cationic radius will influence the lattice enthalpy. Since lattice enthalpy decreases much more than the hydration enthalpy with increasing ionic size, the solubilit y increases as we go down the group.
Question 5.
Why does the solubility of alkaline earth metal carbonates and sulphates in water decrease down the group ?
Solution:
The size of anions being much larger compared to cations, the lattice enthalpy will remain almost constant within a particular group. Since the hydration enthalpies decrease down the group, solubility will decrease as found for alkaline earth metal carbonates and sulphates.