AP State Board Syllabus AP SSC 10th Class Biology Solutions Chapter 2 Respiration – The Energy Releasing System Textbook Questions and Answers.
AP State Syllabus SSC 10th Class Biology Solutions 2nd Lesson Respiration – The Energy Releasing System
10th Class Biology 2nd Lesson Respiration – The Energy Releasing System Textbook Questions and Answers
Improve your learning
Question 1.
Distinguish between
(a) Inspiration and Expiration
(b) Aerobic and Anaerobic respiration
(c) Respiration and Combustion
(d) Photosynthesis and Respiration
Answer:
(a) Inspiration and Expiration:
| Inspiration | Expiration |
| 1. It is also called inhalation. | 1. It is also called exhalation. |
| 2. The air or water is taken into the respiratory organ. | 2. The air or water is sent out of the respiratory organ. |
| 3. It is an active process. | 3. It is a passive process. |
| 4. Rib cage moves forward and outward. | 4. Rib cage moves downward and inward. |
| 5. Diaphragm contracts and becomes flattened. | 5. Diaphragm relaxes and becomes original dome shaped. |
| 6. Increase in volume of thoracic cavity. | 6. Decrease in volume of thoracic cavity. |
| 7. Air pressure in lungs is less than the atmospheric pressure. | 7. Air pressure in lungs is greater than the atmospheric pressure. |
![]()
(b) Aerobic respiration and Anaerobic respiration:
(OR)
Respiration is energy-producing process in the organisms. It takes place both in the presence and absence of oxygen. Laxmi said there are some differences between the two processes. How do you support her?
Answer:
| Aerobic respiration | Anaerobic respiration |
| 1. It takes place in the presence of oxygen. | 1. It takes place in the absence of oxygen. |
| 2. In aerobic respiration, complete oxidation of glucose takes place. | 2. In anaerobic respiration, the glucose molecule is incompletely oxidised. |
| 3. End products are CO2 and water. | 3. End products are either ethyl alcohol or lactic acid and CO2. |
| 4. Lot of energy is liberated (38 ATP). | 4. Relatively small energy is liberated (2 ATP). |
| 5. It occurs in plant’s and animal’s cells. | 5. Occurs in many anaerobic bacteria and human muscle cells. |
6.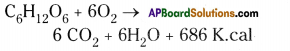 |
6. |
| 7. It has two stages – Glycolysis and Krebs cycle. | 7. It has two stages – Glycolysis and Fermentation. |
(c) Respiration and Combustion:
(OR)
Even though both are oxidation processes, combustion and respiration are different in many aspects. Explain those differences.
(OR)
Combustion and respiration are oxidative processes but still there are differences between them. What are they?
(OR)
Write the differences between respiration and combustion.
Answer:
| Respiration | Combustion | |
| 1. It occurs in living cells. | 1. It is non – cellular. | |
| 2. Oxidation of food materials especially glucose to carbon dioxide and water is called respiration. | 2. When sugar burns CO2 and water are produced and energy is released as heat. This process is called combustion. | |
| 3. Oxidation of sugar molecules occurs at the body temperature of an organism. | 3. Heat is to be supplied for the sugar molecule to burn. | |
| 4. The energy is released in several stages. | 4. The energy is released at once as heat. | |
| 5. Several intermediate substances are formed. | 5. No intermediate substances are formed. | |
| 6. Enzymes are required for oxidation. | 6. Enzymes are not required for combustion. | |
| 7. Respiration occurs in the presence of water. | 7. Combustion occurs in the absence of water. | |
| 8. It is a controlled process. | 8. It is an uncontrolled process. | |
| 9. Energy is stored in ATP in the body. | 9. Energy is not stored and is released into the atmosphere. | |
(d) Photosynthesis and Respiration:
| Photosynthesis | Respiration |
| 1. Occurs only in all plants and photosynthetic bacteria. | 1. Occurs in all living organisms. |
| 2. Takes place in the presence of sunlight. | 2. Takes place throughout day and night. |
| 3. A plant can survive without performing photosynthesis for few days. | 3. No organism can survive without respiration for few minutes even. |
| 4. In plants, only few cells perform photosynthesis. | 4. All living cells in an organism perform this process. |
| 5. Raw materials are C02 and water. | 5. Uses carbohydrates and oxygen. |
| 6. Oxygen is liberated. | 6. Carbon dioxide is released. |
| 7. It occurs in chloroplast. | 7. It takes place in cytoplasm and mitochondria. |
| 8. Adds weight to the organism. | 8. Decrease weight of the organism. |
| 9. It is an anabolic process. | 9. It is a catabolic process. |
10.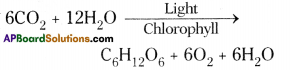 |
10. |
Question 2.
State two similarities between aerobic and anaerobic respiration.
Answer:
Similarities between aerobic and anaerobic respiration:
- Both are catabolic processes.
- Both aerobic and anaerobic respiration takes place in all cells.
- Energy is released in both the processes.
- CO2 is the end product of both processes.
- First stage of both respiration is glycolysis.
- Respiratory substances in both processes are glucose, fatty acids and amino acids.
![]()
Question 3.
Food sometimes enters the wind pipe and causes choking. How does it happen?
Answer:
- Pharynx is the common passage of both air and food.
- From here air enters into trachea and food enters into oesophagus.
- Pharynx is connected to larynx through glottis a slit like opening.
- A cartilagenous flap called EPIGLOTTIS act as a lid over glottis and prevents food from entering into trachea during swallowing.
- Any food particles enters the trachea it causes chocking.
- Sometimes the food particles are forced back by cough.
Question 4.
Why does the rate of breathing increase while walking uphill at a normal pace in the mountains? Give two reasons.
Answer:
The rate of breathing increases while walking uphill at a normal pace in the mountains.
- It is because as we go up the hill above sea level the concentration of oxygen is greatly reduced. So we have to breathe more to get required amount of oxygen.
- While walking uphill a lot of oxygen is used by our body to release energy from glucose.
- This leads to lack of oxygen in the cells.
- We take in oxygen when we breathe.
- Hence to increase the amount of oxygen intake there is an increase in breathing rate during walking uphill.
Question 5.
Air leaves the tiny sacs in the lungs to pass into capillaries. What modification is needed in the statement?
Answer:
- Gaseous exchange takes place within the lungs by diffusion from the alveoli to blood capillaries and vice versa.
- The carbondioxide in the blood is exchanged for oxygen in alveoli.
- This sentence may be modified as “Air that contains oxygen reaches the tiny sacs in the lungs to pass into capillaries.
![]()
Question 6.
Plants photosynthesize during daytime and respire during the night. Do you agree to this statement? Why? Why not?
Answer:
- No, I do not agree with this statement. Plants photosynthesize during daytime only and respire during the daytime as well as night time also.
- During daytime when photosynthesis occurs in the presence of sunlight. Oxygen is produced. The leaves use some of this oxygen for respiration and the rest diffuses into air.
- During daytime CO2 produced by respiration is all used up in photosynthesis by leaves.
- At night time no photosynthesis occurs and oxygen diffuses into leaves to carryout respiration.
Question 7.
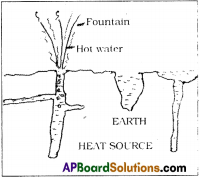
Why does a deep sea diver carry oxygen cylinder on his/her back?
Answer:
- When we go deep into the sea, the oxygen level decreases. Oxygen is in dissolved state in water.
- Humans are adapted for utilizing oxygen in gaseous state. They cannot use dissolved oxygen for breathing.
- Only aquatic animals like fish can utilize the dissolved oxygen for breathing using gills.
- Human beings have lungs for respiration. Therefore, sea divers have to carry oxygen cylinders in their back so as to receive oxygen.
- If they do not carry them, they do not get oxygen and there is a chance even to die.
![]()
Question 8.
How are alveoli designed to maximise the exchange of gases?
Answer:
The human lungs have been designed to maximise the exchange of gases as follows.
- The interior of lung is divided into millions of small chambers called alveoli.
- The presence of millions of alveoli in the lungs provide a very large surface area.
- If all alveoli of our lungs are spread out they will cover an area of nearly 160 m2.
- Availability of large surface area maximises the exchange of gases.
Question 9.
Where will the release of energy from the glucose in respiration take place? Mala writes lungs, while Jiya writes muscles. Who is correct and why?
Answer:
- Respiration is the process of releasing energy from the breakdown of glucose.
- Respiration takes place in every living cell, all the time.
- All cells need to respire in order to produce the energy that they require.
- During respiration the release of energy from the glucose takes place in muscles but not in lungs.
- So Jiya is correct. The energy is released from the muscle cells during respiration. Only gaseous exchange takes place in lungs.
![]()
Question 10.
What is the role of epiglottis and diaphragm in respiration?
Answer:
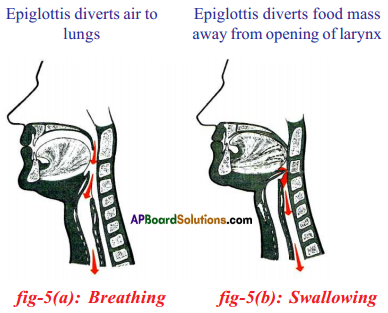
Epiglottis:
- Epiglottis, a flap like muscular valve controls movement of air and food towards their respective passages.
- Epiglottis is partly closed when we swallow food and it opens more widely when we take a breath and air enters the lungs.
- Epiglottis allows air pass through the larynx and the respiratory system.
Diaphragm :
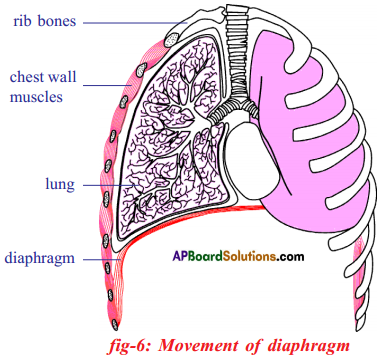
- The diaphragm in the respiratory system is the dome shaped sheet of muscle that separates the chest from the abdomen.
- When the diaphragm contracts during inhalation it flattens out a bit. This results in the enlargement of the volume of the chest cavity.
- This reduces the pressure in the lungs and air enters into lungs from outside the body.
- During exhalation, the diaphragm relaxes and assumes its dome shape. This change increases the pressure on the lungs and squeezes the air through the nose to the atmosphere.
![]()
Question 11.
How does gaseous exchange take place at blood level?
Answer:
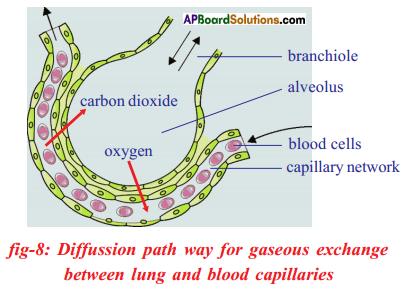
- Lungs are made up of several thousands of small chambers called alveoli.
- Within the alveoli, exchange of gases take place between the gases inside the alveoli and blood.
- Blood arriving in the alveoli has higher CO2 concentration which is produced during respiration by the body cells.
- At the same time air in the alveoli has a much lower concentration of CO2 and this allows the diffusion of CO2 out of the blood and to alveolar air.
- Similarly blood arriving in the alveoli has a lower oxygen concentration while air in the alveoli has a higher oxygen concentration.
- Therefore oxygen moves into the blood by diffusion.
Question 12.
Explain the mechanism of gaseous exchange at bronchiole level.
Answer:
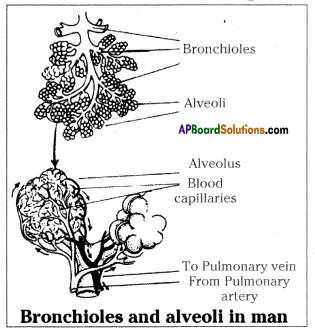
- the trachea (wind pipe) is divided into two tubes called BRONCHI. As there are two lungs each bronchus (singular) enters the lungs on the same side.
- In the lung, the bronchus divides into smaller and smaller branches called BRONCHIOLES which enters into each alveoli.
- When oxygen from outside reaches the alveoli through bronchioles and the carbondioxide from alveoli moves out.
- The inhaled air from outside enters into bronchioles through nostrils → nasal cavities → pharynx → larynx → trachea → bronchus.
- The exhaled air from alveoli enters bronchioles → pharynx → nasal cavities → nostrils → outside.
![]()
Question 13.
After a vigorous exercise or work we feel pain in muscles. What is the relationship between pain and respiration?
Answer:
- We obtain energy by oxidation of glucose molecule.
- In the absence of oxygen (anaerobic respiration) glucose is converted to latic acid.
- During vigorous exercise oxygen gets used up faster in the muscle cells that can be supplied by blood.
- When oxygen supply is inadequate the muscles use energy released during the anaerobic breakdown of glucose.
- The anaerobic respiration by muscles bring about partial breakdown of glucose to form lactic acid.
- The accumulation of lactic acid in the muscles causes muscular pains or cramps.
Question 14.
Raju said, “Stems also respire along with leaves in plants”. Can you support this statement? Give your reasons.
Answer:
Yes. I support the statement of Raju that stems also respire along with leaves in plants.
The reasons are
- The stems of herbaceous plants have stomata.
- So the exchange of respiratory gases in the stems of herbaceous plants takes place through stomata.
- The oxygen from air diffuses into the stem through stomata and reaches all the cells for respiration.
- The carbon dioxide released during respiration diffuses out into the air through the stomata.
- In woody stems the bark has lenticels for gaseous exchange. Through lenticels, oxygen diffuses in and carbon dioxide diffuses out into the air.
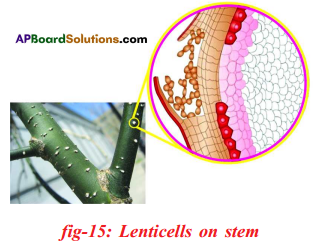
Question 15.
What happens if diaphragm is not there in the body?
Answer:
- The lungs cannot draw in air or push it out by themselves. The chest wall muscles and the diaphragm helps the lungs in moving air into and out of them.
- If diaphragm is not there in the body, we would not be able to breathe.
- The diaphragm is the major muscle in the process of respiration.
- It separates the thoracic and abdominal cavities.
- In the absence of diaphragm, the relaxation and contraction of the chest wall muscles do not take place and thereby inspiration and expiration become difficult that leads to death of the person.
![]()
Question 16.
If you have a chance to meet pulmonologist, what questions are you going to ask about pulmonary respiration?
Answer:
If I have a chance to meet pulmonologist, I would like to ask the following questions:
- What is pulmonary respiration?
- What is the organ involved in pulmonary respiration?
- What is the name of blood vessel that brings deoxygenated blood to lungs?
- What is the name of the blood vessel that carries oxygenated blood from the lungs to the heart?
- Out of the two lungs which one is larger than the other?
- What type of diagnostic test will be performed to assess the function of lungs?
- What is pulmonary edema? How does it occur?
- Can all the diseases of the lungs be cured permanently?
Question 17.
What procedure do you follow to understand anaerobic respiration in your school laboratory?
(OR) (Lab Activity)
Write the procedure and observations of the experiment which you have conducted in your laboratory to prove that CO2 and heat are evolved during anaerobic respiration by using yeast.
(OR)
How do you prove that carbon dioxide is released during anaerobic respiration?
(OR)
How do yeast cells convert glucose solution to CO2 and ethyl alcohol?
Answer:
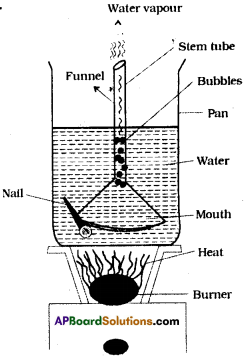
Aim : To prove that CO2 is released during anaerobic respiration.
Apparatus: Thermos flask, splitted corks, thermometer, wash bottle, glass tubes, liquid
paraffin, glucose solution, yeast cells, bicarbonate solution.
Procedure:
- Remove dissolved oxygen from glucose solution by boiling it for a minute and then cooling it without shaking.
- Now add some yeast to the glucose solution and fix a two-holed rubber stopper to the flask.
- The supply of oxygen from the air can be cut off by pouring a 1cm layer of liquid paraffin into the mixture through the holes.
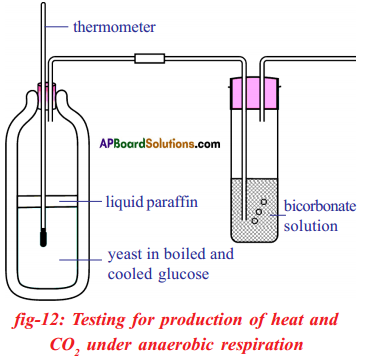
- Insert one end of the thermometer into the thermos flask. See the mercury bulb of thermometer keep inside the solution.
- Arrange for any gas produced by the yeast to escape through a wash bottle containing bicarbonate solution or lime water as shown in the figure.
- Add a few drops of diazine green (Janus Green B) solution to the yeast suspension before you pour liquid paraffin over it.
- The blue diazine green solution turns pink when oxygen is in short supply around it.
- Warm the apparatus to about 37° F in order to speed up the test.
- Keep the apparatus undisturbed for one or two days.
Observations :
- After two days it can be observed that lime-water of the wash bottle turns into milky white precipitate.
- Increase in temperature in thermometer.
- Alcohol smell given off from the flask.
Result: These observations indicate that yeast cells respire anaerobically converting glucose solution into CO2, ethyl alcohol and release heat energy.
![]()
Question 18.
What are your observations in the combustion of sugar activity?
Answer:
Observations in the combustion of sugar:
- When sugar is heated, first it melts, chars and later burns producing flames.
- When sugar is combusted, carbon dioxide and water are produced.
- Energy is also released in the form of heat and it released at once.
- We cannot control the combustion of sugar.
- Intermediate products are not formed.
- We can combust sugar in the absence of water and enzymes.
- When combustion of sugar, heat energy is released into the atmosphere and we cannot store it for further use.
Question 19.
Collect information about cutaneous respiration in frog. Prepare a note and display them in your classroom.
(OR)
How does frog respire with the help of skin?
Answer:
- Respiration through skin is called cutaneous respiration.
- In frog, skin is an additional or secondary or accessory respiratory organ.
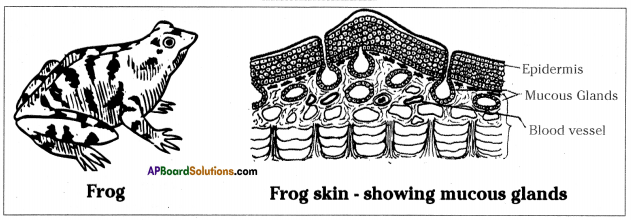
- Skin is a very important respiratory organ in both on land and water.
- One-third of the total oxygen taken up by frog is through the skin.
- Frog also keeps its skin moist. Frog skin has a large number of mucous glands which secrete mucous onto the surface of the skin.
- The mucous layer retains water and reduces evaporation of water from body.
- To keep the skin wet and moist frogs jump into water very frequently.
- Frog skin is supplied with a large number of blood vessels which help in absorbing oxygen from the water.
- The carbon dioxide produced during to respiration, diffuses out into the water through the blood vessels present in the skin of the frog.
![]()
Question 20.
Collect information about respiratory diseases (because of pollution, tobacco) .and discuss with your classmates.
Answer:
Respiratory diseases because of pollution:
- Irritation of eyes, nose, mouth and throat.
- Headaches and dizziness.
- Respiratory symptoms such as coughing and running nose.
- Respiratory and lung diseases including
a) Asthma attacks
b) Chronic obstructive pulmonary disease (COPD)
c) Reduced lung function
d) Pulmonary cancer caused by a series of carcinogen chemicals that through inhalation
e) Mesothelioma: A particular type of lung cancer, usually associated with expo¬sure to asbestos (it usually occurs 20 – 30 years after the initial exposure)
f) Pneumonia: Infection of lungs caused by bacteria.
g) Bronchitis: It is inflammation or swelling of bronchial tubes.
h) Emphysema: It is a lung condition in which tiny air sacs in lungs alveoli fill up with water.
Respiratory diseases due to tobacco :
- Chronic bronchitis: A long term inflammation of the bronchi is characterized by coughing.
- Lung cancer: An abnormal continuous multiplication of cells that can result in tumors in the lining of the bronchi.
- Emphysema: A chronic lung condition that affects the air sacs in the lungs characterized by shortness of breath, coughing, fatigue, sleep and heart problems.
Question 21.
What is the pathway taken by air in the respiratory system? Illustrate with a labelled diagram.
Answer:
The path way taken by air in the respiratory system:
Nostrils → Nasal cavity → Pharynx → Larynx → Trachea → Bronchus → Bronchioles → Alveolus → Blood.
- Nostrils : Air enters the body through the nostrils.
- Nasal cavity: Air is filtered and its temperature is also brought close to that of the body.
- Pharynx: It is the junction of respiratory and digestive system. Epiglottis – a flap like muscular valve controls movement of air and food towards their respective passages.
- Larynx: Also called voice box. This stiff box contains vocal cords. When air passes out of the lungs and over vocal cords, it causes them to vibrate. This produces sounds on the basis of our speech, song etc.
- Trachea: This is also called wind pipe. It channels air to lungs.
- Bronchi: Trachea at it’s lower end divides into two bronchi one leading to each lung.
- Bronchioles: The bronchi further divided into smaller and smaller branches called bronchioles.
- Alveoli: Clusters of air sacs called alveoli in the lungs which are very small and numerous. The gaseous exchange takes place here as blood capillaries take up oxygen and expel CO2.
- Blood: It carries oxygen, to each and every cell of the body and collects CO2 from them.
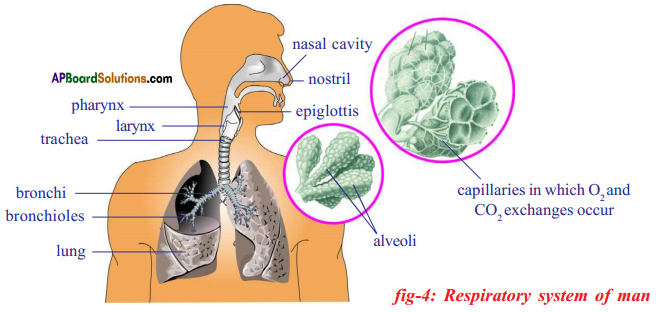
![]()
Question 22.
Draw a block diagram showing events in respiration. Write what you understood about cellular respiration.
Answer:
Events in respiration :
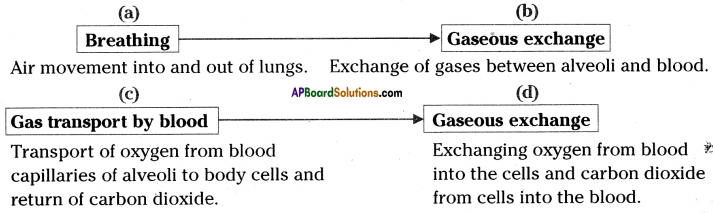

Cellular respiration :
- All living cells must carry out cellular respiration.
- Oxidation of glucose of fatty acids releasing energy takes place in cells, hence it is called cellular respiration.
- It can be in the presence of oxygen that is aerobic respiration or in its absence that is anaerobic respiration (fermentation).
- Cellular respiration in prokaryotic cells like that of bacteria occurs within the cytoplasm.
- In Eukaryotic cells cytoplasm and mitochondria are the site of cellular respiration.
- The energy released in cellular respiration is stored in a special compound called ATP.
- ATP is utilised for carrying out other functions in the cell.
![]()
Question 23.
How do you appreciate the mechanism of respiration in our body?
Answer:
- Respiration is essential for life because it provides energy for carrying out all the life processes which are necessary to keep the organism alive.
- The energy that is obtained from respiration is used to build the organism by way of cell growth, reproduction and cell repair, etc.
- All systems in living beings need energy to survive.
- Respiration helps to expel out the toxic carbon dioxide out of the cells. This CO2 will be utilised by the plants to produce food materials through the process of photosynthesis.
- The respiratory system goes into operation from the movement of our birth and works without ever stopping as long as we live our breath continues.
- During exhalation, the vocal cords in the larynx vibrate to produce sounds and help in speaking as we like.
Question 24.
Prepare an article on anaerobic respiration to present school symposium.
Answer:
Anaerobic respiration :
- Respiration that occurs without oxygen is known as anaerobic respiration.
- It is present in primitive organisms and muscular cells in higher animals.
- Alcohol, CO2 and H2O are end products in this process.
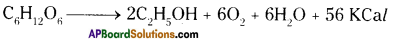
Merits:
- In the absence of oxygen it is good process.
- Mechanism is simple.
- Suitable to microorganisms
Demerits:
- Provides less energy
- Not suitable to higher animals
![]()
Question 25.
Prepare a cartoon on discussion between haemoglobin and chlorophyll about respiration.
Answer:
Discussion between haemoglobin and chlorophyll about respiration:

Haemoglobin: Hello good morning chlorophyll. How are you?
Chlorophyll: Very good morning haemoglobin. I am fine.
Haemoglobin: I am also doing well. Let me know something about you.
Chlorophyll: I am a green coloured pigment present in leaves of plants.
Haemoglobin: How many types of chlorophylls are there ?
Chlorophyll: We are four types i.e., chlorophyll – a, chlorophyll – b, chlorophyll – c and chlorophyll – d.
Haemoglobin: May I know your job in leaves?
Chlorophyll: Yes. Why not? I am an essential factor required to prepare food through the process of photosynthesis in plants.
Haemoglobin: Oh! You are participating in the process of preparing food materials by green plants.
Chlorophyll: Now tell me about your presence.
Haemoglobin: I am present only in animal cells. That too in the red blood cells of the blood. The red colour of the blood is due to my presence.
Chlorophyll: Then tell me about your function in respiration?
Haemoglobin: During respiration, I carry oxygen to the cells in the body tissues.
Chlorophyll: How are you able to do this?
Haemoglobin: I have an oxygen binding element iron. It binds oxygen on four corners of it. I form oxy-haemoglobin with oxygen in the lungs.
Chlorophyll: What happens to the digested food in the cells?
Haemoglobin: In cells, oxygen breakdown the glucose molecule into C02 and H20 releasing large amount of energy. Around 38 ATP molecules are produced.
Chlorophyll: What is the use of this energy?
Haemoglobin: This energy is utilised by the cell to carry other functions.
Chlorophyll: Thank you haemoglobin. You have taught me everything about respiration. In our next meeting we will discuss another topic.
Haemoglobin: Thank you chlorophyll for your interest and patience.
![]()
Fill in the blanks.
- Exhaled air contains ———– and ———–.
- A flap like muscular valve controls movement of air and food is ———–.
- Energy currency of the cell is called ———–.
- Lenticels are the respiratory organs that exist in ———– part of the plant.
- Mangrove trees respire with their ———–.
Answer:
- carbon dioxide, water vapour
- Epiglottis
- ATP (Adenosine Tri Phosphate)
- stem
- aerial roots
Choose the correct answer.
6. We will find vocal cords in [ ]
A) Larynx
B) Pharynx
C) Nasal cavity
D) Trachea
Answer: A
7. Cluster of air sacs in lungs are called [ ]
A) Alveoli
B) Bronchi
Answer: A
8. Which of the following is correct ? [ ]
i) The diaphragm contracts – volume of chest cavity increased
ii) The diaphragm contracts – volume of chest cavity decreased
iii) The diaphragm expands – volume of chest cavity increased
iv) The diaphragm expands – volume of chest cavity decreased
A) i B) i and ii C) ii and iii D) iv
Answer: A
9. Respiration is a catabolic process because of [ ]
A) Breakdown of complex food molecules
B) Conversion of light energy
C) Synthesis of chemical energy
D) Energy storage
Answer: A
10. Energy is stored in [ ]
A) Nucleus
B) Mitochondria
C) Ribosomes
D) Cell wall
Answer: B
10th Class Biology 2nd Lesson Respiration – The Energy Releasing System
Activity – 1
![]()
How do you test the presence of water vapour and heat in the exhaled air?
Answer:
- Keep your palm around an inch away from your nose.
- Feel you breathing out.
- Do not remove your palm until you have finished the activity.
- Breathe steadily for 1 – 2 minutes.
- Now take a piece of any fruit.
- Chew and before swallowing it keep the fingers of the other palm on your neck, now swallow it.
Questions:
- What did you notice? What happens to your breath as you try to swallow?
Answer:
We cannot swallow while breathing. We usually stop breathing when we swallow food. - What is helping you to swallow without deflecting it to the windpipe?
Answer:
Epiglottis is helping me to swallow without deflecting food to the windpipe.
Activity – 2
Write an experiment to observe changes during combustion of sugar.
(OR)
What are your observations in combustion of sugar activity.
Answer:
Aim: To observe changes during combustion of sugar.
Apparatus: Wooden stand, test tubes, rubber stopper, delivery tube, glucose or sucrose powder, lime water, spirit lamp.
Procedure:
- Take a small amount of glucose in a small test tube.
- Arrange the apparatus as shown in the figure.
- Heat the test tube until the glucose catches fire.
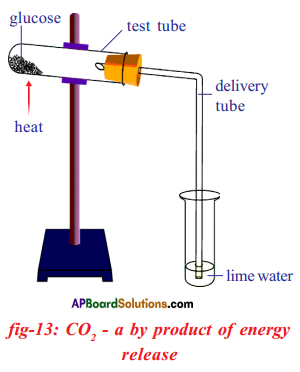
Observations :
- In the initial stage the glucose becomes liquid.
- Later it turns to black colour after catching fire.
- In this process carbon dioxide and water are produced.
- Energy is released as heat.
- The carbon dioxide released changes lime water to milky white.
Conclusion:
- From this experiment, we can conclude that carbon dioxide, water and heat are produced during combustion of glucose in the laboratory.
- The carbon dioxide changes lime water to milky white in nature.
Activity – 3
![]()
How can you prove that carbon dioxide is evolved during respiration?
(OR)
Write the experimental procedure and draw the arrangement of apparatus to show that CO2 is evoloved in respiration.
To understand that CO2 is evolved during respiration, what experiment you have performed in your laboratory? Explain the procedure.
(OR)
Write an experiment to prove that CO2 is released during respiration.
Answer:
Aim: To prove that CO2 is released during aerobic respiration.
Apparatus: Two wide mouthed plastic or glass bottles, germinating seeds, dry seeds, two small injection bottles or beakers with lime water.
- Take two wide mouthed glass bottles.
- Keep germinating bengal gram seeds in one bottle and dry seeds in another bottle.
- Keep two small beakers with lime water in each glass bottles.
- Close the glass bottles tightly.
- Keep both the sets undisturbed for one or two days.
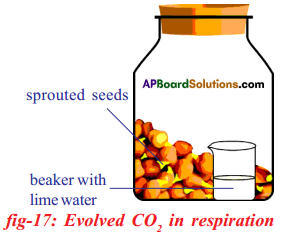
Observation:
- After two days it can be observed that lime water of the beaker placed in the bottle containing germinating seeds turns into milky white.
- And the lime water kept in the glass bottle containing dry seeds do not change its colour.
Result: It indicates that germinating seeds liberated carbon dioxide which turns lime
water into milky white.
Activity – 4
![]()
Explain the procedure you have adopted in your school to prove that heat is liberated during respiration. What result we will get, if you perform this experiment with dry seeds?
(OR)
Write the procedure you have followed to observe “heat is evolved during respiration” in your laboratory. What precautions did you take during the activity?
Answer:
Aim: To prove that heat is liberated during respiration.
Apparatus: Two Thermos flasks, two thermometers, two rubber corks, dry seeds, germinating seeds.
Procedure:
- Take a handful of moong or bazra seeds.
- Soak the seeds in water a day before experiment.
- Keep these soaked seeds in a cloth pouch and tie with a string tightly.
- Next day collect the sprouts / germinated seeds from the pouch in a thermos flask and take dry seeds in another thermos flask.
- Remove the lid and prepare a cork through which you can bore a hole to insert thermometers into two flasks in such a way that the bulb of the thermometer should dip into the germinating and dry seeds.
- Close the thermos flasks with tight fitting rubber corks.
- Record the initial temperature in both the flasks and record it for every two hours for at least 24 hours.
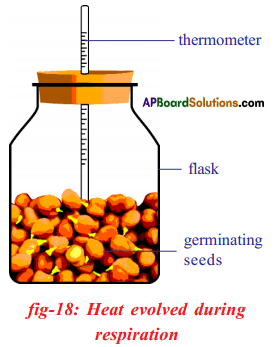
Observation: Constant increase in the temperature is observed in thermometer placed in the germinating seeds.
Result: Hence it is proved that germinated seeds respire and liberate heat which is responsible for increase in the temperature.
Questions:
- Make a graph by using your observations.
Answer:
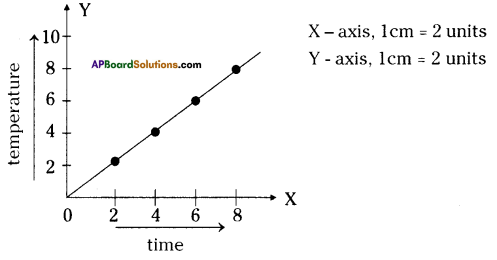
- Is there any increase in temperature?
Answer:
Yes, there is increase in temperature. - Does the temperature increase steadily or does it abruptly increase at a time of the day?
Answer:
The temperature in the thermometer increases steadily. - Where does the heat come from?
Answer:
The sprouting or germinating seeds respire and liberate heat.
10th Class Biology 2nd Lesson Respiration – The Energy Releasing System InText Questions and Answers
10th Class Biology Textbook Page No. 25
![]()
Question 1.
Can it be said that Priestly’s experiment helped us to find out more about composition of air? How?
Answer:
Yes, Priestly’s experiments helped to find out the composition of air when burning of charcoal, carbon dioxide is produced which is the one of the composition of air done by Lavoisier.
Another experiment with phosphorus done by Lavoisier was cleared that a gas which is the respirable air that is helped in burning was oxygen also a component of air.
Lavoisier proved experimentally that carbon dioxide and oxygen were the components of air. Lavoisier confirmed the experiments of Priestly about the gases present in the air.
Question 2.
What gas was produced by combustion according to Lavoisier?
Answer:
The gas produced by combustion is carbon dioxide.
Question 3.
What did Lavoisier find out about air from his experiments?
Answer:
A fixed air carbon dioxide and respirable air oxygen which helped in burning were liberated during his experiments.
Question 4.
What conclusion can be drawn from Lavoisier’s experiments?
A. Oxygen and carbon dioxide are the compositions of air.
10th Class Biology Textbook Page No. 26
![]()
Question 5.
Which gas do you think is Lavoisier talking about when he says chalky acid gas?
Answer:
Carbon dioxide.
Question 6.
Which gas according to Lavoisier is respirable air?
Answer:
Oxygen.
Question 7.
What steps in the process of respiration does Lavoisier mention as an inference of his experiments?
Answer:
Lavoisier mentioned that there were two steps in the respiration.
1. Inspiration: Breathing oxygen.
2. Expiration: Eliminating carbon dioxide from lungs.
Question 8.
It is a common observation that our breath is warmer than the air around us ; does respiration have anything to do with this?
Answer:
Our exhaled air is warmer than the air around us because heat is liberated during respiration.
10th Class Biology Textbook Page No. 27
![]()
Question 9.
What does this experiment indicate?
Answer:
This experiment indicates that carbon dioxide is liberated during respiration.
Question 10.
Which gas turns lime water milky?
Answer:
Carbon dioxide.
Question 11.
Which gas do you think might be present in greater quantities in the air we breathe out as compared to air around us?
Answer:
Nitrogen (78%) is present in greater quantities in the air.
Question 12.
We are also aware of the fact that water vapour deposits on a mirror if we breathe out on it; where does this water vapour come from in Exhaled air?
Answer:
Water vapour is liberated during respiration.
10th Class Biology Textbook Page No. 29
![]()
Question 13.
Why are we advised not to talk while eating food?
Answer:
- When we are eating food, epiglottis helps to avoid food entering into trachea.
- If we talk while we eat food, there is a chance of food entering into trachea and causes choking.
- As a result irritation and inflammation takes place in the respiratory tract.
- So we are advised not to talk while eating.
10th Class Biology Textbook Page No. 30
Question 14.
What is the role of diaphragm and ribs in respiration? Are both active in man and woman?
Answer:
- Diaphragm and ribs are helpful the chest cavity to increase or decrease the volume for inspiration and expiration in respiration.
- Diaphragm plays a major role in men and ribs play a major role in mechanism of respiration.
10th Class Biology Textbook Page No. 31
![]()
Question 15.
What can be concluded from this?
Answer:
All movements of breathing is controlled by nerves leading from the brain.
Question 16.
What happens during the process of breathing?
Answer:
During the process of breathing, the patterns of breathing show a great range for they are coordinated with moment by moment needs of the body for supply of oxygen and removal of carbon dioxide.
Question 17.
Which gas needs to be removed from our body during exhalation? Where does the extra amount of gas come from?
Answer:
Carbon dioxide needs to be removed during exhalation. The extra amount of gas comes from the breakdown of glucose to release energy in the mitochondria. Carbon dioxide gas is released here.
Question 18.
What is the composition of inhaled air?
Answer:
Inhaled air contains oxygen -21%, C09 – 0.03%, Nitrogen – 78%.
![]()
Question 19.
When exhaled air is compared with inhaled air, is there any difference in composition?
Answer:
Yes, there is a difference between inhaled air and exhaled air.
The difference is

10th Class Biology Textbook Page No. 32
Question 20.
Why does the amount of oxygen vary between exhaled and inhaled air?
Answer:
Because some amount of oxygen will be utilised during cellular respiration in the body. Hence the difference in amount of oxygen occurs.
Question 21.
What has raised the percentage of carbon dioxide in exhaled air?
Answer:
CO2 is released from all the cells in the body in respiration and is added to the exhaled air.
10th Class Biology Textbook Page No. 34
![]()
Question 22.
Do cells of alveoli or lungs also require oxygen to carry out cellular respiration? Why / Why not?
Answer:
- Alveoli are made of squamous epithelium tissue which is very thin and elastic
- Alveoli are so thin that oxygen can pass from air-filled alveoli to R.B.C inside the vessels.
- Simple squamous epithelial cells function as mediators of filtration and diffusion. As these cells are living tissue they also need oxygen.
- This is done through the exchange of gases in the alveoli.
Question 23.
After undergoing strenuous exercise we feel pain in muscles, does adequate oxygen reach the muscles?
Answer:
No. Adequate oxygen does not reach the muscles.
Question 24.
What is being formed in the muscles?
Answer:
Lactic acid.
10th Class Biology Textbook Page No. 42
![]()
Question 25.
In which set does the colour change faster? Why?
Answer:
In the set which has germinating seeds the colour changes faster. Because CO2 is formed faster in aerobic respiration.
Think and Discuss
10th Class Biology Textbook Page No. 29
Question 1.
What will happen if the respiratory tract is not moist?
Answer:
- If the respiratory tract is not moist the dirt particles in the inhaled air will not be removed from air in the nasal cavities and reaches lungs and create problems to lungs.
- The temperature of the inhaled air is brought close to that of the body for the smooth passage in the respiratory tract. If it is dry, it is not possible.
- If the surface dries out, gas exchange will happen at a very reduced rate since fast moving gaseous oxygen molecules do not efficiently cross the alveoli membrane.
- The reduced gas exchange is most likely not enough to support blood oxygenation for vital functions of the body.
- Hence respiratory tract should be moist for smooth exchange of gases.
Question 2.
Are both lungs similar in size?
Answer:
No. Right lung is slightly bigger than left lung.
![]()
Question 3.
Why are alveoli so small and uncountable in number?
Answer:
- The pouch-like air sacs at the ends of the smallest bronchioles are called alveoli.
- The walls of the alveolus are very thin and they are surrounded by very thin blood capillaries.
- It is in the alveoli that gaseous exchange takes place.
- There are millions of alveoli in the lungs. The presence of millions of alveoli in the lungs provides a very large area for the exchange of gases.
- And the availability of large surface area maximises the exchanges of gases.


 Answer:
Answer:
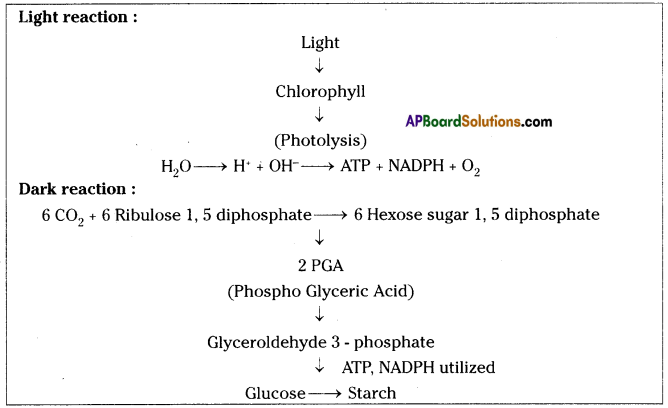
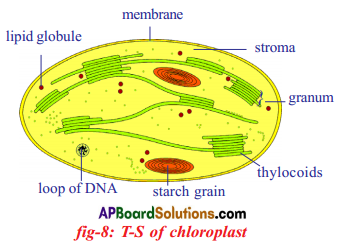
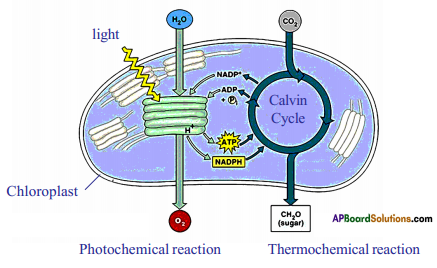
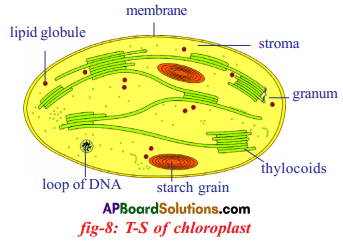 Role of Chloroplast in photosynthesis:
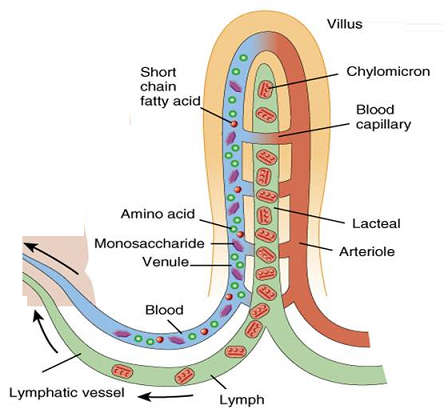
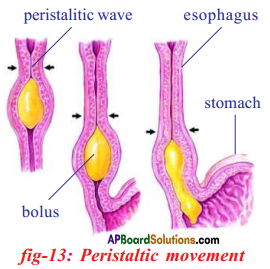
Role of Chloroplast in photosynthesis: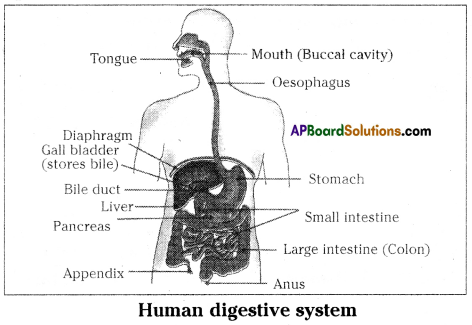 Parts where peristalsis takes place: Oesophagus, stomach, small intestine and large intestine.
Parts where peristalsis takes place: Oesophagus, stomach, small intestine and large intestine.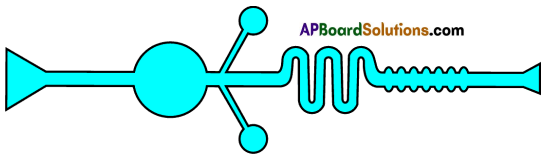 Answer:
Answer: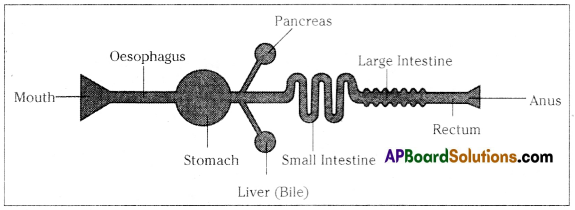
 Answer:
Answer: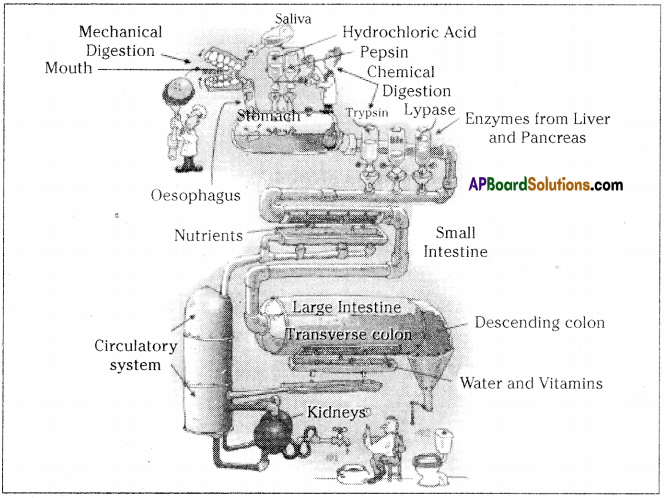
 Apparatus:
Apparatus:
 Priestly should have tilted the bell jar to one side and introduced the mint plant without disturbing the experimental set up.
Priestly should have tilted the bell jar to one side and introduced the mint plant without disturbing the experimental set up.