Andhra Pradesh BIEAP AP Inter 1st Year Chemistry Study Material 3rd Lesson Chemical Bonding and Molecular Structure Textbook Questions and Answers.
AP Inter 1st Year Chemistry Study Material 3rd Lesson Chemical Bonding and Molecular Structure
Very Short Answer Questions
Question 1.
What is Octet rule?
Answer:
Every atom must possess 8 electrons in its outermost energy level for its stability. Atoms combine in two ways to get octets either by transfer of electrons (or) by mutual sharing of electrons. They can attain ns2 np6 configuration.
The tendency of an atom to achieve eight electrons in its outermost shell is known as the octet rule.
Question 2.
Write Lewis dot structures for S and S2-.
Answer:
- Lewis dot structure for ‘s’ is
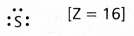
Electronic configuration — 1s2 2s2 2p6 3s2 3p4 - Lewis dot structure for s-2 is
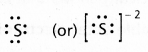
Electronic configuration of s-2 is 1s2 2s2 2p6 3s2 3p6
![]()
Question 3.
Write the possible resonance structures for SO3.
Answer:
The resonance structures of SO3 as follows
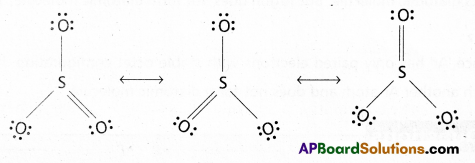
Question 4.
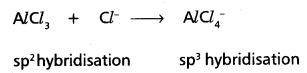
Predict the change, if any, in hybridization of Al atom in the following reaction
AlCl3 + Cl– → \(\mathrm{AlCl}_4^{-}\).
Answer:
In AlCl3 Aluminium undergoes sp2 hybridisation
In \(\mathrm{AlCl}_4^{-}\) Aluminium undergoes sp3 hybridisation
Question 5.
Which of the two ions Ca2+ or Zn2+ is more stable and why ?
Answer:
- Ca+2 has electronic configuration 1s22s22p63s23p6. This configuration is noble gas (or) inert gas configuration.
- Zn+2 has electonic configuration 1s22s22p63s23p64s03d10. This configuration is psuedo inert gas configuration.
∴ Ca+2 is more stable than Zn+2 ion.
Question 6.
Cl– ion is more stable than Cl atom—Why ?
Answer:
The electronic configurations of Cl and Cl– is :
Cl = 1s2 2s2 2p6 3s2 3p5
Cl– = 1s2 2s2 2p6 3s2 3p6.
This electronic configuration clearly shows that chlorine atom has 7 electrons in the outermost orbit. Whereas chloride has a stable octet electronic configuration (3s2 3p6). After gaining one electron, chloride ion attains the electronic configuration of Argon. Hence chloride ion has greater stability than chlorine atom.
Question 7.
Why argon does not form Ar2 molecule ?
Answer:
Ar2 represents diatomic molecule. But Argon does not form diatomic molecule. So it cannot represented as ‘Ar2‘.
Reason : Since Ar’ has only paired electrons with stable octet configuration. It cannot share its electrons with another Ar atom and does not form diatomic molecule.
Question 8.
What is the best possible arrangement of four bond pairs in the valence shell of an atom to minimise repulsions ?
Answer:
The best possible arrangement of four bond pairs in the valency shell of an atom to minimise repulsions is Tetrahedral. (Bond angle 109°.28′)
Eg. : Methane (CH4).
Question 9.
If A and B are two different atoms when does AB molecule become Covalent ?
Answer:
- If the difference in electronegativity values between A and B is less than 1.7, then covalent compound formation is possible (according to Allred – Rochow scale).
- If A and B are sharing one or more electron pairs mutually then AB will be a covalent compound.
![]()
Question 10.
What is meant by localized orbitals?
Answer:
The molecular orbital with bonded electron cloud localised between the two nuclei of bonded atoms is called localized orbital, (or) The orbitals which are involved in bond formation are called localized orbitals.
Question 11.
How many Sigma and Pi bonds are present in
(a) C2H2 and
(b) C2H44?
Answer:
a) C2H2
H – C ≡ C – H
C2H2 contains 3 – sigma bonds and 2 – pi bonds.
b) C2H4
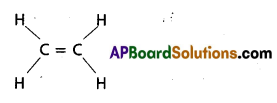
C2H4 contains 5 – sigma bonds and 1-pi bond.
Question 12.
Is there any change in the hybridization of Boron and Nitrogen atoms as a result of the following reaction? BF3 + NH3 → F3BNH3
Answer:
a) Ammonia – Boron trifluoride formation (H3N → BF3):
Ammonia molecule contains Nitrogen atom with a lone pair of electrons (in sp3 orbital). BF3 has ‘B’ atom with an incomplete octet (with a vacant Pz orbital). Therefore, nitrogen of ammonia donates its lone pair to Boron and thus forms coordinate covalent bond. During this bond formation, the sp3 orbital of nitrogen having a lone pair overlaps the vacant ‘p’ orbital of Boron. The equation corresponding to the reaction is written as follows :
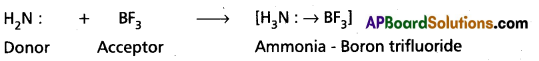
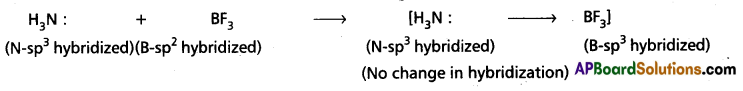
b) Change in hybridised states N and B during [H3N → BF3] formation :
Boron in BF3 undergoes (sp2 hybridization with one vacant unhybrid ‘p’ orbital. This orbital also undergoes) hybridization in presence of NH3 so that the hybridised state of ‘B’ changes from sp2 to sp3. This vacant hybrid orbital is bonded to NH3 through dative bond. During this process there is no change in the hybridized state of Nitrogen in NH3.
Question 13.
Give reasons for the following.
a) Why H2O Boiling point is more than H2S
b) Why H2O Boiling point is more than HF
Answer:
a) H2O has high boiling point than H2S
Reason:
In H2O inter molecular hydrogen bonding is present where as in case of H2S such bonding is absent.
b) H2O has high boiling point than HF
Reason:
In H2O and H2S inter molecular hydrogen bonding is present but in H2O the no. of hydrogen bonds are more than in HF.
![]()
Short Answer Questions
Question 1.
Explain Kossel-Lewis approach to Chemical bonding.
Answer:
Kossel – Lewis Theory : This theory was also called as electronic theory of valency (or) chemical bond theory.
Postulates of Kossel – Lewis Theory : Kossel explained the formation of electrovalent bond while Lewis explained the formation of covalent bond. Their explanation of valency is mainly based on the inertness of noble gases.
Postulates:
- The chemical inertness of noble gases is due to the presence of octet structure. Octet rule was stated as follows “An atom must possess eight electrons in the outermost energy level for its stability”.
- Even though ‘He’ has only two electrons in the valency shell, it is highly stable and chemically inert.
- Elements other than zero group are chemically reactive because of having less than 8 electrons in their outer most shells.
- Every atom try to acquire the Eight electron configuration (octet) in its outer most shell. This can be possible by losing (or) sharing (or) gaining electrons.
- According to Lewis the valency electrons are represented by dots. These are called Lewis symbols.
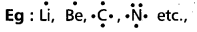
- Lewis dot structures can be used to calculate the group valency of the element.
Eg:
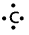 has four electrons.
has four electrons.
∴ Valency of ‘c’ is ‘4’.
Question 2.
Write the general properties of Ionic Compounds.
Answer:
- Physical state : Due to close packing of ions, ionic compounds are crystalline solids.
- Melting and Boiling points : In ionic crystals the oppositely charged ions are bound by strong electrostatic force of attraction. To overcome these attractive force between ions, more thermal energy is required. Hence the melting and boiling points of ionic compounds are high.
- Solubility : Ionic compounds are soluble in polar solvents like water, liquid ammonia etc., but are insoluble in non – polar solvents like benzene, carbon disulphide etc.,
- Reactivity: Reactions between ionic compounds in aqueous solution are very fast due to strong attraction among ions.
e.g. : When AgNO3 solution is added to NaCl solution, a white precipitate of AgCl is formed.
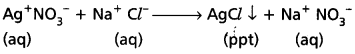
- Isomerism : Ionic bond is non-directional.
So ionic compounds cannot exhibit isomerism. - Electrical conductivity : Ionic substances conduct electricity in molten state and in aqueous solution. The ionic compounds are, therefore, electrolytes.
![]()
Question 3.
State Fajan’s rules and give suitable examples.
Answer:
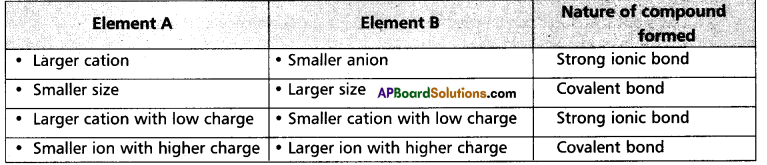
Fajan’s rules:
- Ionic nature of the bond increases with increase in the size of cation, e.g.: The ionic nature increases in the order
Li+ < Na+ < K+ < Rb– < Cs+ - The formation of ionic bond is favoured with the decrease of the size of anion.
e.g.: CaF2 is more ionic than CaI2. - If the charge on cation (or) anion (or) both is less, then they can form ionic bonds, e.g.: The ionic nature increases in the order
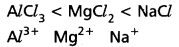
- Cations with inert gas configurations form ionic compounds while those cations with pseudo inert gas configurations favour covalent bond formation.
e.g.: Na+ in Na+Cl– has an inert gas configuration. So Na+Cl– is ionic. But CuCl is more covalent because Cu+ has not acquired inert gas configuration in this compound, instead it has acquired pseudo inert gas configuration. - The cation with inert gas configuration is more stable, e.g.: Ca2+ is more stable than Zn2+ ion.
Question 4.
What is Octet rule ? Briefly explain its significance and limitations.
Answer:
Octet rule: Every atom must possess 8 electrons in its outermost energy level for its stability. Atoms combine in two ways to get octet either by transfer of electrons (or) by mutual sharing of electrons. They can attain ns2 np6 configuration.
e.g.:
- Na loose one electron to get Ne configuration by possessing 8 electrons.
Na : 1 s2 2s2 2p6 3s1 and Na+ : 1 s2 2s2 2p6. - ‘Cl’ atom take one electron to get “Ar” configuration. Cl– : 1s2 2s2 2p6 3s2 3p6.
Here Na+ and Cl– ions obey octet rule. - In H2O molecule oxygen obey octet rule.
It is therefore, concluded that s2p6 configuration in the outer energy level constitutes a structure of maximum stability and therefore, of minimum energy.
The atoms of all elements when enter into chemical combination try to attain noble gas configuration (i.e.,) they try to attain 8 electrons in their outermost energy level which is of maximum stability and hence of minimum energy.
The tendency of an atoms to achieve eight electrons in their outermost shell is known as OCTET RULE.
Octet rule was the basis of electronic theory of valency.
Limitations : There are 3 types of exceptions to the octet rule. These are mentional below.
- Central atoms containing incomplete octet.
Eg : BeH2, BCl3 etc., - Molecules containing odd number of electrons.
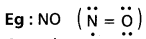
- Central atoms possessing more than 8 electrons which istermed as expanded octet.
Eg : SF6, H2SO4 etc., - This theory does not explained about shape of molecules.
- This theory does not explained about the formation of noble gas compounds like XeF2, XeOF2 etc.,
Question 5.
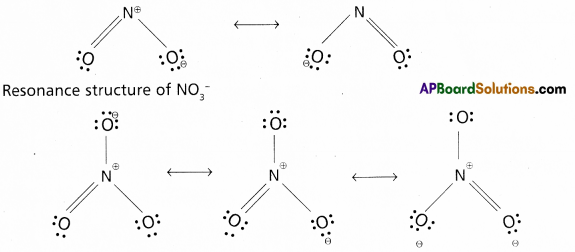
Write the resonance structures for NO2 and \(\mathrm{NO}_3^{-}\)
Answer:
Resonance structure of NO2
Question 6.
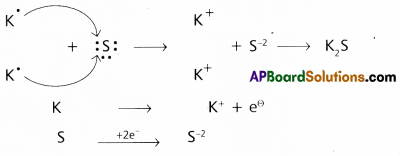
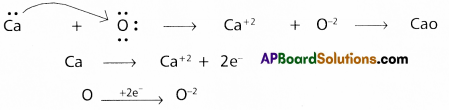
Use Lewis symbols to show electron transfer between the following pairs of atoms to form cations and anions:
(a) K and S
(b) Ca and O
(c) Al and N.
Answer:
a) Between the atoms K and S
b) Between Ca and O
c) Between Al and N
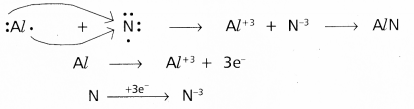
Question 7.
Explain why H2O has dipole moment while CO2 does not have.
Answer:
- H2O molecule is a polar molecule and it has un symmetrical structure i.e. Angular (or) V – shape
- CO2 molecule is non polar and it is linear molecule.
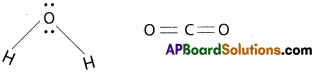
- SO H2O has dipolemoment (μ = 1 ,835D) and CO2 does not have dipolemoment (μ = 0)
Question 8.
Define Dipole moment. Write its applications.
Answer:
Dipole moment : The product of magnitude of the charge and the distance between the two poles (bond length) is called dipole moment.
- Dipole moment μ = q × d
q = charge
d = bond length - Units : Debye (D), 1 Debye = 3.34 × 10-30 coulombs metres.
Applications : –
- Dipole moment is used to calculate the percentage of ionic character in a molecule.
- It is used to know the shape of the molecule.
- Symmetry (symmetrical (or) non symmetrical) of the molecule can be known by dipole moment.
![]()
Question 9.
Explain why BeF2 molecule has zero dipole moment although the Be-F bonds are polar.
Answer:
- Even though Be-F bonds in BeF2 are polar, the dipole moment of BeF2 molecule is zero. Because BeF2 has linear shape.
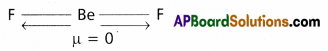
- Flere the vectrorial sum of the dipole moment of two Be-F bonds is zero. Hence dipole moment
(μ) = 0.
Question 10.
Explain the structure of CH4 molecule.
Answer:
Formation of Methane molecule :
- The central atom of methane is carbon.
- The electronic configuration of carbon in ground state is
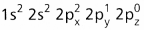 and on excitation it is
and on excitation it is 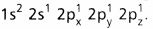 During excitation the 2s pair splits and the electron jumps into the adjacent vacant 2pz orbital.
During excitation the 2s pair splits and the electron jumps into the adjacent vacant 2pz orbital. - The
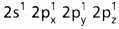 undergo sp3 hybridisation giving four equivalent sp3 hybridised orbitals.
undergo sp3 hybridisation giving four equivalent sp3 hybridised orbitals. - Each sp3 hybrid orbital overlaps with the 1s orbitals of hydrogen forming \(\sigma_{s p^3-s}\) bond.
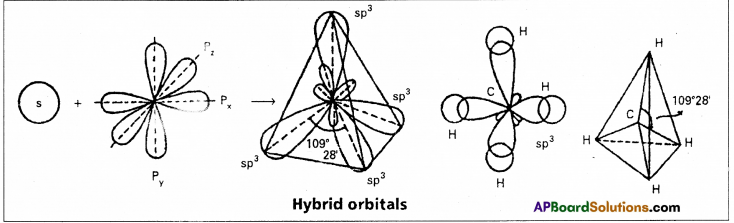
- In case of methane four \(\sigma_{s p^3-s}\) bonds are formed. The bonds are directed towards the four corners of a regular tetrahedron. The shape of methane molecule is tetrahedral with a bond angle 109°28’.
Question 11.
Explain Polar Covalent bond with a suitable example.
Answer:
The covalent bond which is formed by the mutual sharing of electron pairs between two dissimilar atoms is called polar covalent bond.
- Dissimilar atoms means two atoms having different electronegativity values (or) atoms of different elements.
Eg : HF, HCl, H2O, CO2 etc.,
Formation of HCl :
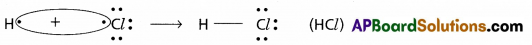
- In the above example H and Cl are two atoms of different elements having different electro negativities.
- These two atoms (H, Cl) mutually share the electron pairs and form the polar covalent bond.
Question 12.
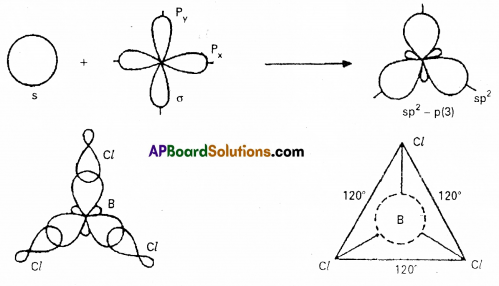
Explain the shape and bond angle In BCl3 molecule in terms of Valence Bond Theory.
Answer:
Boron trichloride molecule formation :
- The electronic configuration of ‘B’ in the ground state is
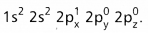
- On excitation the configuration is
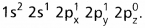 Now there are three half filled orbitals are available for hybridisation.
Now there are three half filled orbitals are available for hybridisation. - Now sp2 hybridisation takes place at boron atom giving three sp2 hybrid orbitals.
- Each of them with one unpaired electron forms a ‘σ’ bond with one chlorine atom. The overlapping is σsp2 – p (Cl atom has the unpaired electron in 2pz orbital). In boron trichloride there are three ‘σ’ bonds.
Question 13.
What are σ and π bonds ? Specify the differences between them.
Answer:
Definition of σ bond : “σ – bond is along the internuclear axis. It has a cylindrical symmetry”.
Definition of π bond : “A covalent bond formed by a sidewise overlap of ‘p’ orbitals of atoms that are already bonded through a σ – bond, and in which the electron clouds are present above and below the internuclear axis is known as π – bond”.
Sigma bond (σ)
- A ‘σ’ bond is formed by the axial overlap of two half filled orbitals belonging to the valence shells of the two combining atoms.
- The ‘σ’ bonding electron cloud is symmetric about the inter-nuclear axis.
- It is strong bond since the extent of overlap is much.
- It allows free rotation of atoms or groups about the bond.
- It can exist independently.
- It determines the shape of the molecule.
- There can be only one ‘σ’ bond between two atoms.
- Hybrid orbitaIs form only ‘σ’ bonds.
Pi — bond (π)
- A π-bond is formed by the lateral overlap of orbitals.
- The π-bonding electron cloud lies above and below the flame of the internuclear axis.
- It is a weaker than ‘σ’ bond, since the extent of overlap is less.
- π-bond restricts such free rotation.
- It is formed only after a ‘σ’ bond is formed.
- It does not determine the shape of the molecule.
- There can be one or two π-bonds between the atoms.
- Hybrid orbitals cannot form π-bonds.
![]()
Question 14.
Even though nitrogen in ammonia is in sp3 hybridization, the bond angle deviate from 109°28. Explain.
Answer:
In NH3 molecule the central nitrogen atom shares its ‘3’ unpaired electrons with three hydrogen atoms to form 3σ bonds. Hence NH3 molecule contains one lone pair, three bond pairs.
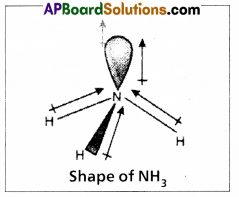
a) Because of the repulsion between the lone pair and the bond pairs the angle reduces to 107°.
b) According to VSEPR theory the geometry of the molecule is pyramidal with bond angle 107°.
Question 15.
Show how a double and triple bond are formed between carbon atoms in
(a) C2H4 and
(b) C2H2 respectively.
Answer:
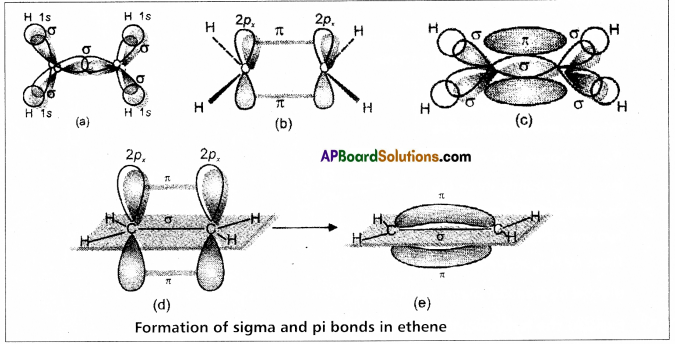
Formation of double bond between the carbon atoms of C2H4 : –
C – Ground state electronic configuration 1s2 2s2 2p2
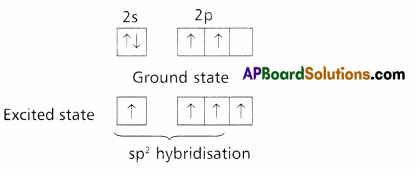
- In ethylene two carbons undergo sp2 hybridisation.
- One of sp2 hybrid orbital of carbon overlaps with sp2 hybrid orbital of another carbon atom to form C – C sigma bond.
- The two other sp2 hybrid orbitals of each carbon overlap with ‘s’ orbital of hydrogen atoms to form C – H bonds.
The unhybridised orbital of one carbon atom overlap side wisely with the similar orbital to form weak π bond.
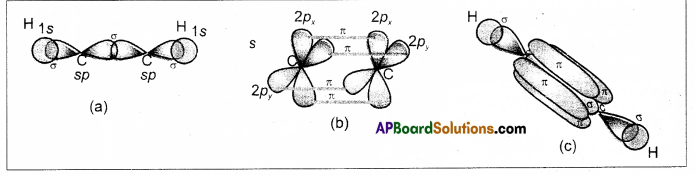
Formation of triple bond between the carbon atoms of C2H2 : –
C – Ground state electronic configuration 1s2 2s2 2p2
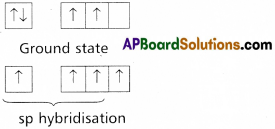
- In acetylene two carbons undergoes sp hybridisation.
- One of sp hybrid orbital of carbon overlaps with sp hybrid orbital of another carbon atom to form C – C sigma bond.
- another sp hybrid orbitals of each carbon overlap with s orbital of hydrogen atoms to form C – H bonds.
- The un hybridised orbitals of two carbon atom overlap side wisely to form two weak π bonds.
Question 16.
Explain the hybridization involved in PCl5 molecule. (T.S. Mar. ’16, ’15)
Answer:
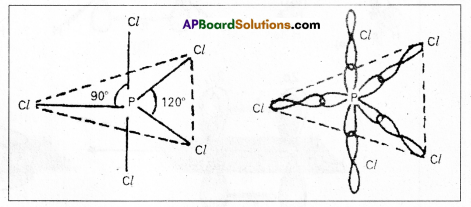
1) In PCl5 the electron configuration of phosphorus is
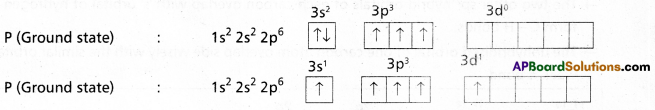
2) Phosphorus undergoes sp3d – hybridisation by intermixing of one s-orbital [3s], three p – orbitals [3px, 3py, 3pz] and one d – orbital.
These five hybrid orbitals overlap. The pz orbitals of chlorine atoms forming five \(\sigma_{s p^3 d-s}\) bonds. Out of these five p – Cl bonds three are coplanar and the remaining two are in the axial position. There by PCl5 acquires the trigonal bipyramidal shape. The molecule contains two bond angles 90° and 120°.
Question 17.
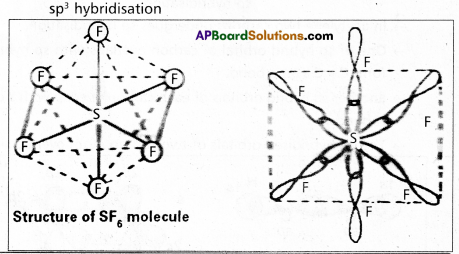
Explain the hybridization involved in SF6 molecule.
Answer:
In this hybridisation one ‘s’ orbital, three ‘p’ orbitals and two ‘d’ orbitals of the excited atom combine to form six equivalent sp3d2 hybrid orbitals.
e.g. : SF6
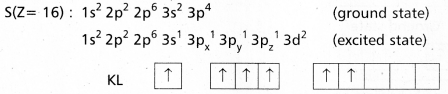
These six sp3 d2 hybrid orbitals overlap six 2pz orbitals of fluorine atoms to form six \(\sigma_{s p^3 d^2}\) bonds. The directions of the bonds give an octa-hedral shape to the molecule. The bond angle is 90° or 180° & 90°.
![]()
Question 18.
Explain the formation of Coordinate Covalent bond with one example.
Answer:
Co-ordinate covalent bond (dative bond) is a special type of covalent bond. It is proposed by Sidgwick. It is formed by the sharing of electrons between two atoms in which both the electrons of the shared electron pair are contributed by one atom and the other atom nearly participates in sharing.
The bond is represented as (“→”) an arrow starting from the donar atom and directed towards the acceptor atom.
Examples :
1) Ammonia – Boron trifluoride H3N : → BF3
Ammonia combines with boron trifluoride to give ammonium boron trifluoride.
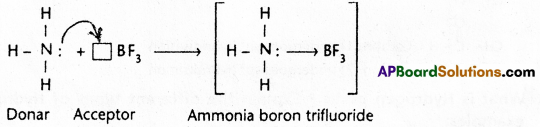
In ammonia nitrogen has a complete octet and also it has a lone pair of electrons. In BF3 the boron atom has a total of six electrons after sharing with fluorine. Nitrogen donates the electron pair to boron to form a co-ordinate covalent bond between ammonia and boron trifluoride.
2) Ammonium ion (\(\mathrm{NH}_4^{+}\))
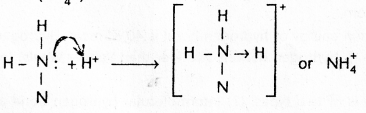
3) Hydronium ion (\(\mathrm{H}_3 \mathrm{O}^{+}\))
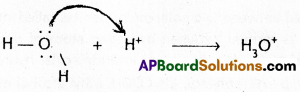
Properties of co-ordinate covalent bond :
- The bond do not ionise in water.
- The compounds are generally soluble in organic solvents and are sparingly soluble in water.
- These compounds exhibit space isomerism because the bond is rigid and directional.
- The bond is semipolar in nature – so their volatility lies in between covalent and ionic bonds.
Question 19.
Which hybrid orbitals are used by Carbon atoms in the following molecules?
(a) CH3 -CH3
(b) CH3 – CH = CH2
(c) CH3 – CH2 – OH
(d) CH3 – CHO
Answer:
a) CH3 -CH3 (ethane)
The two carbons of ethane undergo sp3 hybridisation.
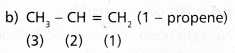
Carbon – (1) – undergoes sp2 hyrbidisation
Carbon – (2) – undergoes sp2 hyrbidisation
Carbon – (3) – undergoes sp3 hyrbidisation
![]()
Carbon (1) and (2) both undergo ‘sp3‘ hybridisation.

Question 20.
What is Hydrogen bond ? Explain the different types of Hydrogen bonds with examples. (A.P., T.S. Mar. ’16)
Answer:
Hydrogen bond is a weak electrostatic bond formed between partially positive charged hydrogen atom and an highly electronegative atom of the same molecule or another molecule.
Hydrogen bond is formed when the Hydrogen is bonded to small, highly electronegative atoms like F, O and N. A partial positive charge will be on hydrogen atom and partial negative charge on the electronegative atom.
The bond dissociation energy of hydrogen bond is 40 KJ/mole. Hydrogen bond is represented with dotted lines (—–). Hydrogen bond is stronger than Vander Waals’ forces and weaker than covalent bond.
Hydrogen bonding is of two types.
(1) Intermolecular hydrogen bond and
(2) Intramolecular hydrogen bond.
1) Intermolecular hydrogen bond :
If the hydrogen bond is formed between two polar molecules it is called intermolecular hydrogen bond, i.e., the hydrogen bond is formed between hydrogen atom of one molecule and highly electronegative atom of another molecule is known as intermolecular hydrogen bond.
Ex. : Water (H2O) ; HF : NH3 ; p – nitrophenol, CH3COOH, ethyl alcohol etc.
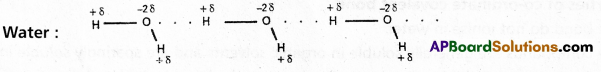
Water molecule forms oi. associated molecule through intermolecular hydrogen bond. Due to molecular association water possess high boiling point.
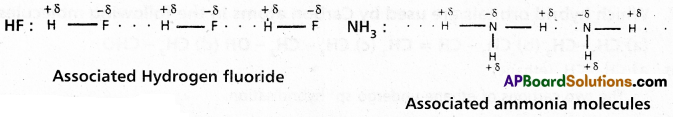
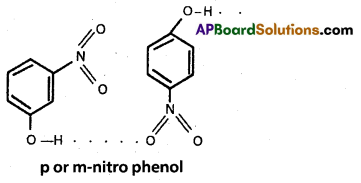
m or p – nitrophenol :
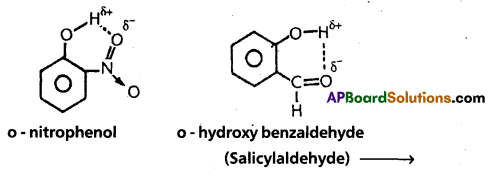
2) Intramolecular hydrogen bond:
If the hydrogen bond is formed within the molecule it is known as intramolecular hydrogen bond.
Ex. : o – nitrophenol; o – hydroxy benzaldehyde.
Abnormal behaviour due to hydrogen bond:
- The physical state of substance may alter. They have high melting and boiling points.
- Ammonia has higher boiling point than HCl eventhough nitrogen and chlorine have same electronegativity values (3.0). Ammonia forms an associated molecule through intermolecular hydrogen bond.
- p – hydroxy benzaldehyde have higher boiling point than o- hydroxy benzaldehyde. This is due to intermolecular hydrogen bonding in para isomer.
- Ethyl alcohol is highly soluble in water due to association and co-association through intermolecular hydrogen bonding.
![]()
Question 21.
Explain the formation of H2 molecule on the basis of Valence Bond theory.
Answer:
Postulates of valency bond theory:
- Covalent bond is formed by the overlap of an half filled atomic orbital of one atom with an half filled atomic orbital of the other atom involved in the bond formation.
- The electrons in these two orbitals involved in the overlap shall have opposite spins.
- Greater the overlap stronger the bond formed.
- The bonds are formed mostly in the direction in which the electron clouds are concentrated.
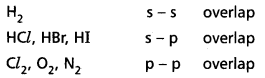
Formation of H2 molecule:
Hydrogen molecule is formed due to overlaping of s – s orbitals. When two Hydrogen atoms come together, is orbitals of the Hydrogen atoms overlap to form a strong ‘σ” bond. This is σs-s’
Question 22.
Using Molecular Orbital Theory explain why the B2 molecule is paramagnetic ?
Answer:
Boron electronic configuration is – 1s2 2s2 2p1
The molecular orbital energy level sequence for B2 is
![]()
- Bond order = \(\frac{6-4}{2}\) = \(\frac{2}{2}\) = 1
- In the above sequence unpaired electrons are present.
- Presence of unpaired electrons leads to paramagnetic nature.
∴ B2 molecule is paramagnetic.
Question 23.
Write the important conditions necessary for linear combination of atomic orbitals.
Answer:
- The molecular orbitals are formed when the atomic orbitals combine linearly (i.e.,) when the atoms approach each other. The no. of molecular orbitals resulting are equal to the no.of atomic orbitals combining.
- Only such atomic orbitals which are of similar energies and symmetry with respect to the inter nuclear axis combine to form molecular orbitals.
- The total no. of molecular orbitals produced will be numerically equal to the no. of combining orbitals.
- The order of energies of bonding, anti bonding and non bonding orbitals can be written as bonding orbitals < non bonding orbitals < anti – bonding orbitals.
Question 24.
What is meant by the term Bond order? Calculate the bond orders in the following
(a) N2
(b) O2
(c) \(\mathrm{O}_2^{+}\) and
(d) \(\mathrm{O}_2^{-}\)
Answer:
Bond order : The half of the difference between the no.of bonding electrons and anti bonding electrons is known as bond order. ’
a) N2 : Molecular orbital energy level sequence.
![]()
→ Bond Order = \(\frac{10-4}{2}\) = \(\frac{6}{2}\) = 3
b) O2 : Molecular orbital energy level sequence.
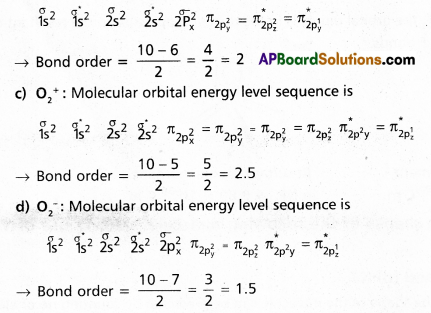
![]()
Question 25.
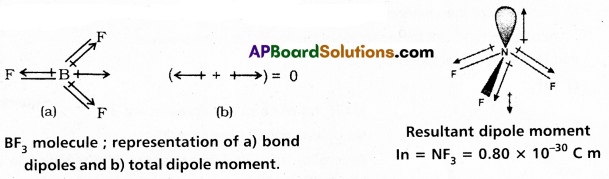
Of BF3 and NF3, dipole moment is observed for NF3 and not for BF3. Why ?
Answer:
Of BF3 and NF3 dipole moment is observed for NF3 and not for BF3.
Reasons:
- BF3 molecule is non polar and is a symmetrical molecule. Symmetrical molecules have zero dipole moment.
- NF3 molecule is polar and it is a unsymmetrical molecule so it has dipole moment.
- BF3 molecule has trigonal planar structure.
NF3 molecule has pyramidal shape. - NF3 has dipole moment µ = 0.8 × 10-30 coloumb × meter.
Question 26.
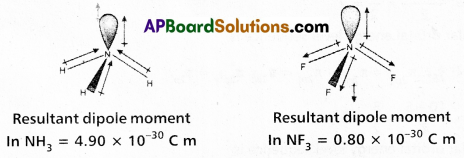
Eventhough both NH3 and NF3 are Pyramidal, NH3 has a higher dipole moment compared to NF3. Why? (A.P. Mar.’16)
Answer:
- Both NH3 and NF3 molecules have pyramidal shape and in two molecules N atom has lone pair of electrons.
- Even though fluorine has more electronegativity than nitrogen the dipole moment of NH3 is greater than that of NF3
µ (NH3) = 4.9 × 10-30 coloumbs × meter.
µ (NF3) = 0.8 × 10-30 coloumbs × meter. - In case of NH3 the orbital dipole due to lone pair is in the same direction as the resultant dipole moment of N – H bonds.
Where as in case of NF3 the orbital dipole is in the direction opposite to the resultant dipole
moment of the three N – F bonds.
Question 27.
How do you predict the shapes of the following molecules making use of VSEPR Theory ?
(a) XeF4
(b) BrF5
(c) ClF3 and
(d) IC\(l_4^{-}\)
Answer:
According to VSEPR theory the shape of the molecule can be predicted by counting no.of electron pairs (bond pairs, lone pairs) around the central atom.
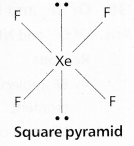
a) XeF4:
In XeF4 No.of bond pairs present are ‘4’.
No.of lone pairs present are ‘2’.
According to VSEPR theory shape of molecule is square planar (Actual shape octahedral).
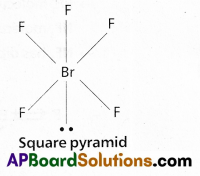
b) BrF5 :
In BrF5 No.of bond pairs present are ‘5’
No.of lone pairs present are ‘1’
According to VSEPR theory shape of the molecule is square pyramid (actual shape octahedral)
c) ClF3:
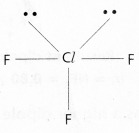
In ClF3 No.of bond pairs present are ‘3’
No.of lone pairs present are ‘2’
According to VSEPR Theory shape of ClF3 Molecule is T – shape (Actual shape TBP)
d) IC\(l_4^{-}\):
In IC\(l_4^{-}\) No.of bond pairs present are ‘4’
No.of lone pairs present are ‘2’
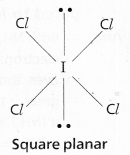
According to VSEPR Theory shape of ICl\(l_4^{-}\) is square planar
(Actual shape octahedral)
Long Answer Questions
Question 1.
Explain the formation of Ionic Bond with a suitable example.
Answer:
The electrostatic force that binds the oppositely charged ions which are formed by the transfer of electrons from one atom with low ionization potential to the other with high electron affinity is called ionic bond or electrovalent bond.
It is formed when the electronegativity difference between the two atoms is more tha 1.7.
Example :
Formation of sodium chloride in terms of orbital concept:
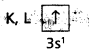
1) Na (Z = 11). The electronic configuration is 1s2 2s2 2p6 3s1.
This can be expressed as
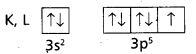
2) Cl (Z = 17). The electronic configuration is = 1s2 2s2 2p6 3s2 3p5
This can be expressed as
3) The configurations after the transfer of electrons forming ions can be expressed as:
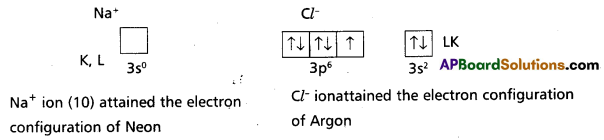
In the formation of sodium chloride the 3s electron of sodium atom is transferred to the 3p2 orbital of chlorine atom. The Na+ ion and Cl– ion so formed are now bound by strong coulombic electrostatic forces of attraction forming sodium chloride.
![]()
Question 2.
Explain the factors favourable for the formation of Ionic Compounds.
Answer:
Factors favour the ionic bond formation:
a) Cation formation:
- Lower ionization energy: Lower ionization energy of an atom greater is the ease of formation of cation
e.g. : The ionization energy of sodium is 117.9 kcal/mole and that of potassium is 100 kcal/mole So K+ ion can readily form than Na+ ion. - Large size of the atom : Large atoms can easily lose the valence electrons. If the size large, the distance between the nucleus and the valence electrons is more and so the force of attraction is less. Therefore the electron can be removed easily from the atom forming cation.
- Ion with lower charge: Small magnitude of charge favours the formation of ions easily.
e.g. : The ease of ion formation increases in the order Na+ > Mg2+ > Al3+. - Cations with inert gas configuration : Ion possessing electronic configuration similar to zero group elements are more stable than those ions which do not have such configuration.
eg.: Ca2+ (2, 8, 8) is more stable than Zn2+ (2, 8, 18) because the former has inert gas
configuration.
b) Anion formation:
- High electron affinity: If the electron affinity of an element is high its anion can be easily formed.
e.g.: Cl + e— → Cl– - Smaller size of atom : Smaller the size of the atom lesser is the distance between the nucleus and the valence orbit. Hence the nuclear attraction on incoming electron is more. So the anion is readily formed.
- Lower charge : Ions with lower charge are more readily formed than those with higher charge.
e.g.: Cl– > O 2- > N3- - The ions with inert gas electronic configuration are more readily formed than others with the same charge. .
c) If the two bonded atoms differ by more than 1.70 in their EN values, the bond between them is ionic in nature.
Question 3.
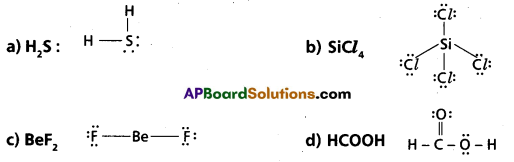
Draw Lewis Structures for the following molecules.
(a) H2S
(b) SiCl4
(c) BeF2
(d) HCOOH
Answer:
Lewis structures:
Question 4.
Write notes on
(a) Bond Angle
(b) Bond Enthalpy
(c) Bond length and
(d) Bond order.
Answer:
a) Bond angle : The angle between the orbitals containing bonding electron pairs around the central atom in a molecule (or) complex ion is known as Bond angle.
- it is expressed in degrees.
- It is determined experimentally by spectroscopic methods.
Eg: In H2O (H — 0 — H) bond angle is 104.5° .
b) Bond Enthalpy : The amount of energy required to break one mole of bonds of a particular type between two atoms in a gaseous state is known as Bond Enthalpy.
Units: KJ/Mole.
Eg: H – H bond enthalpy in hydrogen is 435.8 KJ/mole
H2(g) → H(g) + H(g) ∆H = 435.8 KJ/mole
- In case of poly atomic molecules average bond enthalpy is used.

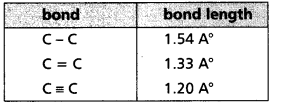
c) Bond length : The distance between the nuclei of the atoms in a molecule is known as bond length.
- Bond length is equal to the sum of the covalent radii of the two atoms that are bonded.
- Units of Bond length A° (or) cm (or) m (or) pm
- As the number of bonds between two atoms increases the bond length decrease.
d) Bond Order:
According to Lewis the bond order is given by the number of bonds between the two atoms in a covalent molecule.
Eg: Bond order of N2 – 3
Bond order of O2 – 2
Bond order of H2 – 1
- In case of Iso electronic species and ions bond orders are same.
- Bond order is useful in predecting stabilities of molecules.
- Bond order increases bond enthalpy increases and Bond length decreases.
![]()
Question 5.
Give an account of VSEPR Theory and its applications.
Answer:
VSEPR theory was proposed by Sidgwick and Powell and later extended by Gillespie and Nyholm. It was developed by Ronald and Nyholm.
This theory explains the shapes of simple molecules having electron pairs bonded or non-bonded. The repulsions among the electron pairs present in the valence shell of the central atom decides the shape of the molecules.
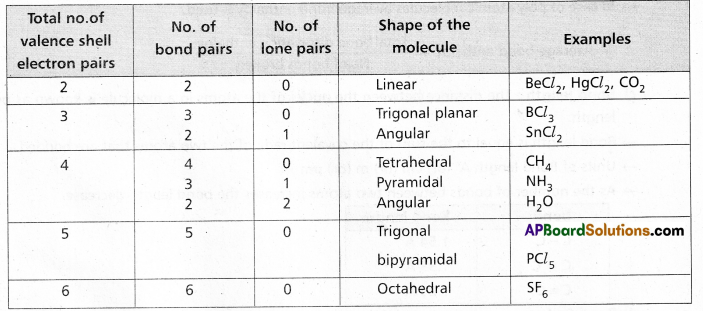
According to this theory :
a) The shape of the molecule is determined by repulsions between all of the electron pairs present in the valency shell of central atom.
b) The electron pairs orient in space so as to have minimum repulsions among them.
c) The magnitude of repulsions between bonding pairs of electrons depends on the electronegativity difference between the central atom and the other atoms.
d) The order of repulsions between various electron pairs is lone pair – lone pair > lone pair – bond pair > bond pair – bond pair.
e) The repulsive forces between different bonds is of the order triple bond > double bond > single bond.
f) The shapes of molecules can be predicted as.
In NH3 molecule the central nitrogen atom shares its ‘3’ unpaired electrons with three hydrogen atoms to form 3σ bonds. Hence NH3 molecule contains one lone pair, three bond pairs.
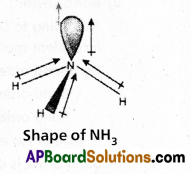
a) Because of the repulsion between the lone pair and the bond pairs the angle reduces to 107°.
b) According to VSEPR theory the geometry of the molecule is pyramidal with bond angle 107°.
Question 6.
How do you explain the geometry of the molecules on the basis of Valence bond Theory ?
Answer:
Postulates of valency bond theory :
- Covalent bond is formed by the overlap of an half filled atomic orbital of one atom with an half filled atomic orbital of the other atom involved in the bond formation.
- The electrons in these two orbitals involved in the overlap shall have opposite spins.
- Greater the overlap stronger the bond formed.
- The bonds are formed mostly in the direction in which the electron clouds are concentrated.
Formation of H2 molecule :
Hydrogen molecule is formed due to overlapping of s – s orbitals. When two Hydrogen atoms come together, 1s orbitals of the Hydrogen atoms overlap to form a strong “σ” bond. This is σs-s.
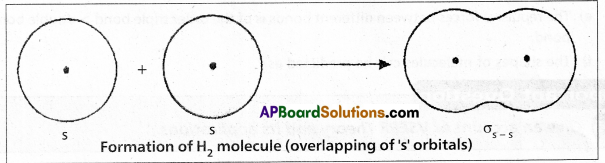
Formation of Cl2 molecule :
The electronic configuration of Chlorine atom is ![]() It has one half filled 3pz orbital. The pz orbital of one chlorine atom overlaps the pz orbital of the other chlorine atom and the two electrons of opposite spins pair up to form covalent bond. As the overlap along the internuclear axis is maximum a strong bond is formed. The bond is formed due to \(\sigma_{p-p}\) overlap.
It has one half filled 3pz orbital. The pz orbital of one chlorine atom overlaps the pz orbital of the other chlorine atom and the two electrons of opposite spins pair up to form covalent bond. As the overlap along the internuclear axis is maximum a strong bond is formed. The bond is formed due to \(\sigma_{p-p}\) overlap.
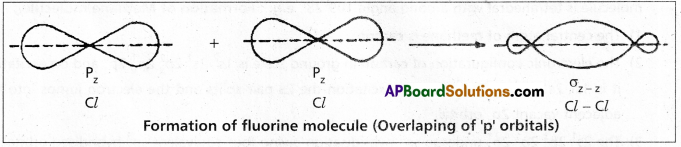
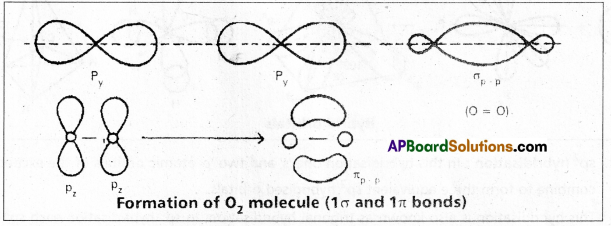
Formation of O2 molecule:
The electronic configuration of oxygen atom is ![]() It has two half filled 2p orbitals i.e., 2py and 2pz.
It has two half filled 2p orbitals i.e., 2py and 2pz.
The py orbital of one atom overlaps the py orbital of the second atom to form a ‘σ’ bond \(\sigma_{p_y}-p_y\).
The pz orbital in the two atoms will be at right angles to the internuclear axis. These two can have lateral overlap. The electron density of the bonded pair is distributed in two banana like regions lying on either side of the internuclear axis. Thus the oxygen molecule has a double bond. The molecule has one σp – p and one πp – p between the two atoms.
Question 7.
‘What do you understand by Hybridisation? Explain different types of hybridization involving s and p orbitals. (Mar. ’13)
Answer:
Hybridisation is defined as the process of mixing of atomic orbitals of nearly equal energy of an atom to give the same number of new set of orbitals of equal energy and shapes.
Depending on the number and nature of orbitals involving hybridisation it is classified into different types. If ‘s’ and ‘p’ atomic orbitals are involved three types are possible namely sp3, sp2 and sp.
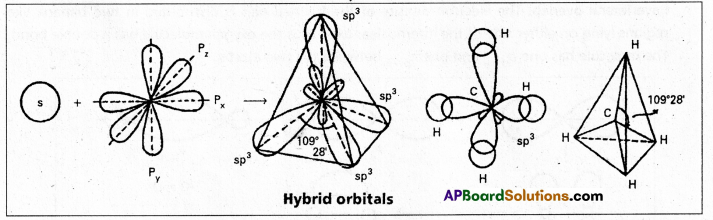
1. sp3 hybridisation : In this hybridisation one’s and three ‘p’ atomic orbitals of the excited atom combine to form four equivalent sp3 hybridised orbitals.
This hybridisation is known as tetrahedral or tetragonal hybridisation.
Each sp3 hybridised orbital possess 25% ‘s’ nature and 75% of ‘p’ nature. The shape of the molecule is tetrahedral with a bond angle 109°28′, e.g. : Formation of Methane molecule :
- The central atom of methane is carbon.
- The electronic configuration of carbon in ground state is
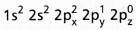 and on excitation it is
and on excitation it is 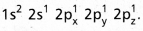 During excitation the 2s pair splits and the electron jumps into the adjacent vacant 2pz orbital.
During excitation the 2s pair splits and the electron jumps into the adjacent vacant 2pz orbital. - The
 undergo sp3 hybridisation giving four equivalent sp3 hybridised orbitals.
undergo sp3 hybridisation giving four equivalent sp3 hybridised orbitals. - Each sp3 hybrid orbital overlaps with the 1s orbitals of hydrogen forming \(\sigma_{s p^3-s}\) bond.
- In case of methane four \(\sigma_{s p^3}-s\) bonds are formed. The bonds are directed towards the four corners of a regular tetrahedron. The shape of methane molecule is tetrahedral with a bond angle 109°28′.
2. sp2 hybridisation : In this hybridisation one ‘s’ and two p’ atomic orbitals of the excited atom
combine to form three equivalent sp2 hybridised orbitals.
This hybridisation is also known as trigorial hybridisation. In sp2 hybridisation each sp2 hybrid orbital has 33.33% ‘s’ nature and 66.66% ‘p’ nature. The shape of the molecule is trigonal with a bond angle 120°.
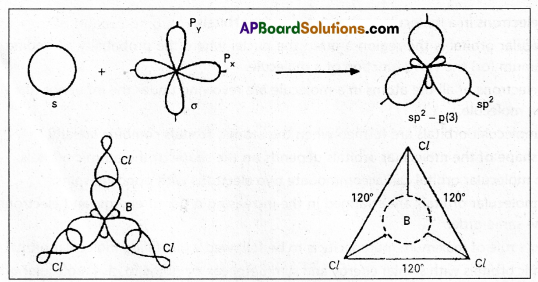
E.g.: Boron trichioride molecule formation:
- The electronic configuration of ‘B’ in the ground state is
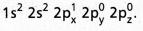
- On excitation the configuration is
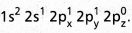 Now there are three half filled orbitals are available for hybridisation.
Now there are three half filled orbitals are available for hybridisation. - Now sp2 hybridisation takes place at boron atom giving three sp2 hybrid orbitals.
- Each of them with one unpaired electron forms ‘σ’ bond with one chlorine atom. The overlapping is \(\sigma_{s p^2-p}\) (Cl atom has the unpaired electron in 2Pz orbital). In boron trichloride there are three ‘σ’ bonds.
3. sp hybridisation : In this hybridisation one ‘s and one ‘p’ atomic orbitals of the excited atom combine to form two equivalent sp hybridised orbitals.
This hybridisation is also known as diagonal hybridisation. In sp hybridisation each sp hybrid orbital has 50% ‘s’ character and 50% ‘p’ character. The shape of the molecule is linear or diagonal with a bond angle 180°.
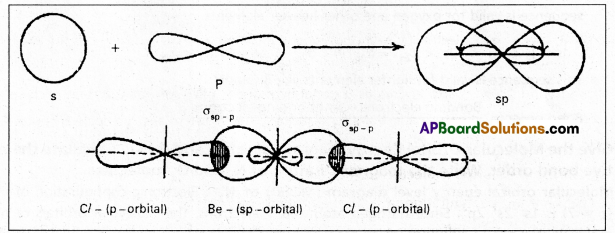
Ex. : Beryllium chloride molecule formation:
- Be atom has
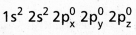 electronic configuration.
electronic configuration. - In ground state it has no half filled orbitais. On excitation the configuration becomes \(1 s^2 2 s^1 2 P_x^1\)\(2 p_y^0 2 p_z^0\).
- Now sp hybridisation takes place at beryllium atom giving two sp hybrid orbitais. Each of them with one unpaired electron forms a ‘σ’ bond with one chlorine atom.
- The overlaping is σsp-p (Cl atom has the unpaired electron in 2pz orbital). In beryllium chloride there are two ‘σ’ bonds.
![]()
Question 8.
Write the salient features of Molecular Orbital Theory.
Answer:
Molecular orbital theory:
Hund and Mulliken.
- Theory was proposed by
- Atomic orbitals (AO) of the bonded atoms combine loose their identity to form molecular orbitals (MO).
- The electrons in a molecule reside in molecular orbitals.
- Molecular orbital is the region around the nuclei where the probability of finding electon is maximum (or) the wave function of a molecule.
- The electrons of all the atoms in a molecule are revolving under the influence of all the nuclei in the molecule.
- The molecular orbitals are formed when the atomic orbitals combine linearly.
- The shape of the molecular orbitals depends on the shape of the atomic orbitals.
- Each molecular orbital can accommodate two electrons with opposite spins.
- The molecular orbitals are arranged in the increasing order of energy, and electrons are filled in the same order.
- Hund’s rule of maximum multiplicity is to be followed while filling molecular orbitals.
- Atomic orbitals with similar energy and symmetry can combine to give molecular orbitals.
- Molecular orbitals with energy lower than A.O are known as bonding molecular orbital; while those with higher energy are known as anti bonding molecular orbitals. Those which are not involved in combination are called non bonding orbitals.
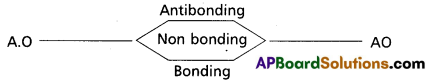
- The order of energies of molecular orbital is : bonding < nonbonding < antibonding molecular orbitals.
- The bonding orbitals are designated a σ and π.
- The antibonding orbitals are designated σ* and π*.
Filling of electrons into molecular orbitals :
The sequence of energy levels of molecular orbitals is given by
![]()
sequence is valid for oxygen and other heavier elements.
![]()
This sequence is valid for lighter elements like B.CandN.
![]()
Question 9.
Give the Molecular Orbital Energy diagram of
(a) N2 and
(b) O2. Calculate the respec- five bond order. Write the magnetic nature of N2 and O2 molecules.
Answer:
Molecular orbital energy level diagram (MOED) of ‘N2‘ ; Electronic configuration of nitrogen (z = 7) is 1s2 2s2 2p3. Since nitrogen atom has 7 electrons, the molecular orbitals of nitrogen molecule (N2) has 14 electrons which are distributed as below :
![]()
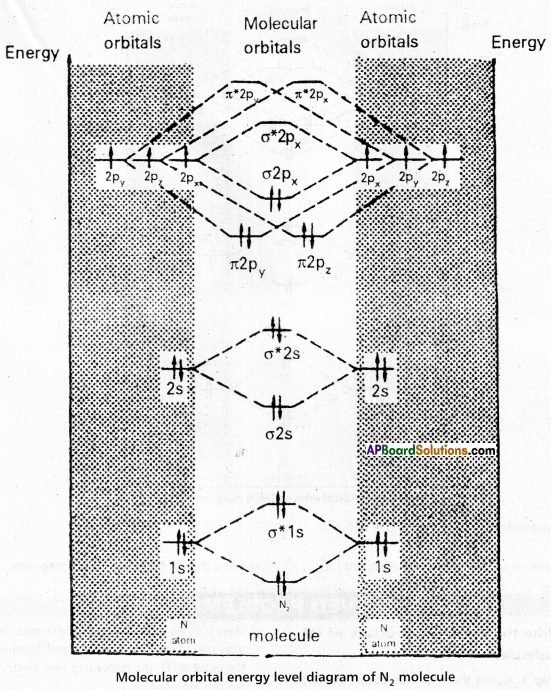
- Bond order = \(\frac{8-2}{2}\) = 3 ( N ≡ N)
- Absence of unpaired electrons showed that N2 molecule is diamagnetic.
MOED of O2:
Electronic configuration of Oxygen (Z = 8) is 1s2 2s2 2p4. Since Oxygen atom has 8 electrons, the molecular orbitais of Oxygen molecule (O2) has 16 electrons, which are distributed as below:
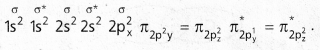
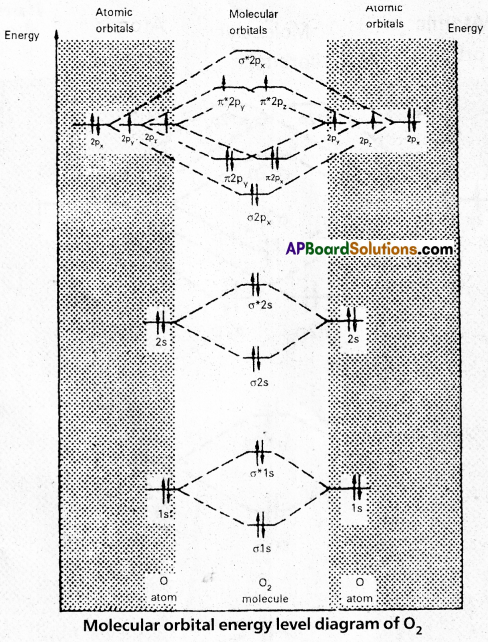
- Bond order = \(\frac{10-6}{2}\) = 2 (O = O)
- Presence of two unpaired 6 electrons \(\left(\pi_{2 p_y^1}^{\star}, \pi_{2 p_z^1}^{\star}\right)\) showed that O2 molecule is paramagnetic.
Solved Problems
Question 1.
Write the Lewis dot structure of CO molecule.
Solution:
Step 1. Count the total number of valence electrons of carbon and oxygen atoms. The outer (valence) shell configurations of carbon and oxygen atoms are: 2s2 2p2 and 2s2 2p4, respectively. The valence electrons available are 4 + 6 = 10.
Step 2. The skeletal structure of CO is written as: C O
Step 3. Draw a single bond (one shared electron pair) between C and O and complete the octet on O, the remaining two electrons are the lone pair on C.
![]()
This does not complete the octet on carbon and hence we have to resort to multiple bonding (in this case a triple bond) between C and O atoms. This satisfies the octet rule condition for both atoms.
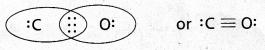
Question 2.
Write the Lewis structure of the nitrite ion, \(\mathrm{NO}_2^{-}\).
Solution:
Step 1. Count the total number of valence electrons of the nitrogen atom, the oxygen atoms and the additional one negative charge (equal to one electron).
N(2s2 2p3), O (2s2 2p4)
5 + (2 × 6) + 1 = 18 electrons
Step 2. The skeletal structure of \(\mathrm{NO}_2^{-}\) is written as: O N O
Step 3. Draw a single bond (one shared electron pair) between the nitrogen and each of the oxygen atoms completing the octets on oxygen atoms. This, however, does not complete the octet on nitrogen if the remaining two electrons constitute lone pair on it.

Hence we have to resort to multiple bonding between nitrogen and one of the oxygen atoms (in this case a double bond). This leads to the following Lewis dot structures.
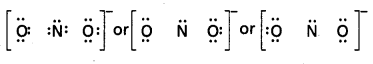
![]()
Question 3.
Explain the structure of \(\mathrm{CO}_3^{2-}\) ion interms of resonance.
Solution:
The single Lewis structure based on the presence of two single bonds and one double bond between carbon and oxygen atoms is inadequate to represent the molecule accurately as it represents unequal bonds. According to the experimental findings, all carbon to oxygen bonds in CCO2 are equivalent.
Therefore the carbonate ion is best described as a resonance hybrid of the canonical forms I, II, and III shown below.
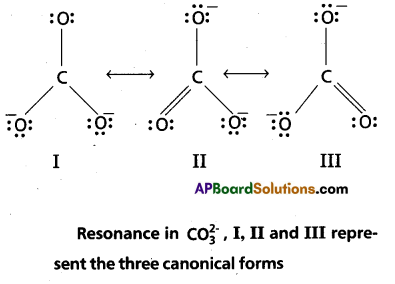
Question 4.
Explain the structure of CO2 molecule.
Solution:
The experimentally determined carbon to oxygen bond length in CO2 is 115 pm. The lengths of a normal carbon to oxygen double bond (C = O) and carbon to oxygen triple bond(C ≡ O) are 121 pm and 110 pm respectively. The carbon-oxygen bond lengths in CO2 (115 pm) lie between the values for C = O and C ≡ O. Obviously, a single Lewis structure cannot depict this position and it becomes necessary to write more than one Lewis structures and to consider that the structure of CO2 is best described as a hybrid of the canonical or resonance forms I, II and III.
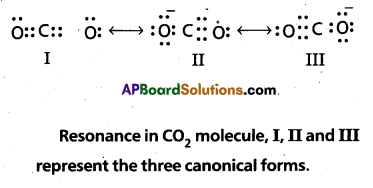
Additional Problems
Question 1.
The experimental dipole moment of HCl is 1.03D and its bond length (distance) is 1.27Å. Calculate the % of ionic character of HCl.
Answer:
Calculated dipole moment = q × d
= 4.8 × 10-10 × 1.27 × 10-8 cm
= 6.09 Debye
% of ionic character = \(\frac{\mu_{\text {ods }}}{\mu_{\text {calc }}}\) × 100
= \(\frac{1.03}{6.09}\) × 100
= 16.9%
Question 2.
The dipole moment of H2S is 0.95D. Find the bond moment if the bond angle is 97° (Cos 48.5° = 0.662).
Answer:
\(\mu_{\text {obs }}\) = 2 (bond moment) \(\left(\cos \frac{\theta}{2}\right)\)
0.95 = 2 (bond moment) (Cos 48.5°)
0.95 = 2 × bond moment × 0.662
Bond moment = \(\frac{0.95}{2 \times 0.662}\) = 0.72D