SCERT AP 7th Class Science Study Material Pdf 2nd Lesson Nature of Substances Textbook Questions and Answers.
AP State Syllabus 7th Class Science 2nd Lesson Questions and Answers Nature of Substances
7th Class Science 2nd Lesson Nature of Substances Textbook Questions and Answers
Improve Your Learning
I. Fill in the blanks.
1. Taste of an acid is ______ .
2. The pH of a substance is 0. It indicates that the substance is ______ in nature.
3. Blue litmus paper turns ______ colour in tamarind solution.
4. The nature of an antacid is ______
5. Acid + base → ______ + ______
Answer:
1. sour
2. acidic
3. red
4. basic
5. salt + water
II. Choose the correct answer.
1. Colour of Turmeric solution in acids
a) Blue
b) Red
c) Purple
d) No change
Answer:
d) No change
2. Example to an acid.
a) Vinegar
b) Baking soda
c) Caustic soda
d) None
Answer:
a) Vinegar
3. Main component of soap is
a) An Acid
b) A base
c) a & b
d) None
Answer:
b) A base
![]()
4. If you add baking soda to lemon juice ______ gas will release.
a) Hydrogen
b) Oxygen
c) Carbon Dioxide
d) Sulphur Dioxide
5. To treat acidic nature of the soil, farmers add ______ to his agricultural field.
a) lemon juice
b) calcium oxide
c) sodium chloride
d) sulphur dioxide
Answer:
b) calcium oxide
III. Matching
| A) Battery | 1. Preservation |
| B) Soap | 2. Calcium carbonate |
| C) Acetic acid | 3. Sulphuric acid |
| D) Hibiscus | 4. Synthetic indicator |
| E) Egg shell | 5. Sodium hydroxide |
| 6. Natural indicator |
Answer:
| A) Battery | 3. Sulphuric acid |
| B) Soap | 5. Sodium hydroxide |
| C) Acetic acid | 1. Preservation |
| D) Hibiscus | 6. Natural indicator |
| E) Egg shell | 2. Calcium carbonate |
IV. Answer the following questions.
Question 1.
Write differences between acids and bases.
Answer:
| Acids | Bases |
| 1) Acids are sour to taste | 1) Bases are bitter to taste |
| 2) They are not slippery to touch. | 2) They are slippery to touch |
| 3) Blue litmus turns red in acids. | 3) Red litmus turns blue in bases. |
| 4) Methyl orange turns red in acids. | 4) Methyl orange turns yellow in bases. |
| 5) Phenolphthalein does not change its colour in acids. | 5) Phenolphthalein turns pink in bases. |
| 6) Acids turns into pink on adding hibiscus indicator. | 6) Bases turns green on adding hibiscus indicator |
| 7) pH of acids is less than 7. | 7) pH of bases is more than 7. |
| 8) Acids react with metals and release hydrogen gas. | 8) Bases like sodium hydroxide react with metals and releases hydrogen gas along with salt. |
Question 2.
Give examples to different types of acid and base indicators.
Answer:
Substances which are used to test acids or bases are called acid base indicators. There are many types of indicators, some of them are
- Natural indicators
- Synthetic indicators
- Olfactory indicators
- Universal indicators.
1) Natural indicators:
Indicators obtained from natural sources are called as Natural indicators. Ex: litmus, turmeric, hibiscus, red cabbage etc.
2) Synthetic indicators:
An indicator prepared from artificial sources is known as Syn-thetic indicator. We use many synthetic indicators like methyl orange, phenolphtha-lein, in our laboratory.
3) Olfactory indicators:
Substances which change their smell when mixed with acid or base are known as olfactory indicators. Ex: onion, vanilla and clove oil.
4) Universal indicators:
It is a mixture of different indicators. It shows different colours in different solutions, “universal indicator” contains thymol blue, methyl red, bromothymol blue and phenolphthalein.
Question 3.
One substance is slippery to touch and bitter in taste, another substance is sour in taste. If you add both, which substances will form?
Answer:
- Substance that is slippery to touch and bitter in taste is Base.
- Substance that is sour in taste is Acid.
- When acids react with base neutralisation reaction takes place.
- Salt and water will form in neutralisation reaction.
Acid + Base → Salt + Water
![]()
Question 4.
Guess, How do you test acetic acid, if there is no availability of indicators?
Answer:
- Acetic acid is a colour less liquid with pungent smell and sour taste.
- It is used in preservation of pickles and other food substances as it is sour.
- So, It can be identify by testing its smell and taste.
Question 5.
Anitha’s mother was filled mango pickle in a ceramic jar and stored, so many doubts were arisen her mind. What would be those doubts?
Answer:
- Why do pickles store only in ceramic jars?
- What materials are used in making ceramic jars?
- What will happen if pickles are stored in metal containers?
- Sometimes pickles spoil quickly. Why?
- Do all pickles contain oil?
- Why is it not suggestable to use aluminium spoons for pickles?
Question 6.
To conduct a neutralization reaction, what materials are required?
Answer:
Materials required to conduct a neutralization reaction are dilute hydrochloric acid (any acid), Sodium hydroxide solution (any base), Phenolphthalein indicator, Conical flask, Dropper.
Question 7.
Draw a neat diagram of arrangement of apparatus to conduct an activity that acid reacts with metal and liberates hydrogen gas.
Answer:
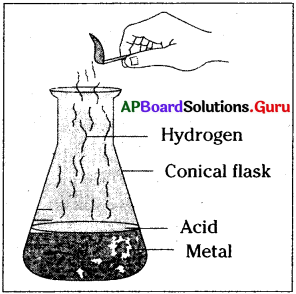
Question 8.
How do you appreciate the role of bases in helping of the persons suffering from acidity problems?
Answer:
- Our stomach produces gastric juice with hydrochloric acid.
- It helps us in digestion of food.
- But sometimes secretion of excess acid causes acidity or indigestion.
- It leaves a burning sensation and pain in the stomach.
- Antacids help us to get relief from acidity.
- Antacids contain bases, eg: aluminum hydroxide, milk of magnesia.
- The bases in the antacids neutralize gastric juice and give us relief.
- Thus bases do a great help to the persons suffering from acidity problems.
Question 9.
What measures do you follow to prevent acid rains?
Answer:
Air pollution is a major cause of acid rains. So acid rains can be prevented by controlling the air pollution. To control air pollution
- Reduce the usage of personal vehicles.
- Reduce the usage of fossil fuels.
- Encourage the usage of alternative sources of energy.
- Use electric vehicles.
- Use solar energy.
- Minimize use of fire and fire products.
- Treat and purify the industrial emissions before letting in to air.
- Plantation and reforestation should be done as much as possible.
7th Class Science 2nd Lesson Nature of Substances InText Questions and Answers
7th Class Science Textbook Page No. 20
Question 1.
Name any other substance which tastes like tamarind?
Answer:
Lemon juice is another substance which has sour taste like tamarind.
Question 2.
Why are tamarind and lemon juice sour in taste?
Answer:
Substances like tamarind and lemon contain some substances which give sour taste. These type of substances with sour taste are known as acids.
7th Class Science Textbook Page No. 21
Question 3.
What do you observe when an acid is poured on the bathroom floor?
Answer:
Generally, hydrochloric acid is used for bathroom cleaning. It liberates thick fumes and pungent smell when poured on the floor.
Question 4.
Have you seen batteries being used in vehicles? Which chemical is used in batteries?
Answer:
Sulphuric acid is used in batteries.
Question 5.
Have you ever drunk a soda or a cool drink?
Answer:
Yes. It contain carbonic acids.
![]()
Question 6.
Which substances do you use to wash your hands and clean your teeth?
Answer:
We use soap to wash our hands and for bathing. We use tooth paste to clean our teeth.
Question 7.
What are the chemicals present in soap and tooth paste?
Answer:
Soap and tooth paste contain bases.
7th Class Science Textbook Page No. 22
Question 8.
See the main components on the tooth paste tube. What chemicals names do you find?
Answer:
Calcium carbonate, aluminium hydroxide and sodium bicarbonate etc.
Question 9.
Is there any substance other than an acid and a base? Discuss with your Mends.
Answer:
The substance which is neither an acid nor a base is known as neutral substance. Pure water is a neutral substance.
Question 10.
List out some acids, bases and neutral substances available in your house.
Answer:
Acids:
Vinegar, lemon juice, tomato juice, bathroom cleaner (hydrochloric acid), cool drinks, curd, etc.
Bases :
Bath soap, cloth soap, baking soda, tooth paste, dish wash liquid etc.
Neutral: Water
7th Class Science Textbook Page No. 23
Question 11.
Is it possible to test all the acids and bases simply by tasting or touching them?
Answer:
- Not possible.
- Some acids like hydrochloric acid, sulphuric acid etc. are very harmful and strong acids. We cannot test them by touch or taste.
Question 12.
In such cases, how can we test them whether they are acids or bases?
Answer:
By using acid base indicators.
Question 13.
What are the different types of indicators?
Answer:
There are many types of indicators, some of them are
- Natural indicators
- Synthetic indicators
- Olfactory indicators
- Universal indicators.
7th Class Science Textbook Page No. 24
Question 14.
Can we use any flower as an indicator?
Answer:
We can use hibiscus flowers as an indicator. We can also use Indigofera tinctoria (Neeli chettu) flowers as indicator.
7th Class Science Textbook Page No. 26
Question 15.
Why it is so? What makes the hydrochloric acid dangerous than vinegar?
Answer:
- Hydrochloric is an strong acid whereas vinegar is a weak acid.
- pH of hydrochloric is very less than that of vinegar.
Question 16.
How can we measure the strength of an acid or a base?
Answer:
Strength of acid or base solutions is measured in pH scale.
7th Class Science Textbook Page No. 27
Question 17.
What happens when a metal piece is dropped in an acid?
Answer:
If you drop a metal piece in an acid it reacts with acid and releases hydrogen gas.
7th Class Science Textbook Page No. 28
Question 18.
Pickles are not stored in aluminium or steel or copper vessels. Why?
Answer:
- Pickles contain acids.
- These acids react with metal containers and releases toxic substances.
- So, we should not store them in metal containers.
- Generally, they are stored in ceramic or glass containers which are not react.
Question 19.
What will happen when metals are placed in bases?
Answer:
Bases like sodium hydroxide react with metals and release hydrogen gas.
7th Class Science Textbook Page No. 29
Question 20.
What will happen if we mix an acid and a base?
Answer:
If we add an acid to a base, they neutralize each other.
7th Class Science Textbook Page No. 30
Question 21.
How is acidity caused? What is the remedy for it?
Answer:
- Excess secretion of gastric juice (hydrochloric acrd) in the stomach causes acidity problems.
- Antacids help the patients to get relief from acidity.
![]()
Question 22.
What are antacids?
Answer:
Antacids are bases. They neutralize the gastric juice in the stomach.
7th Class Science Textbook Page No. 31
Question 23.
How can you help the farmers in finding out whether their agriculture fields are acidic or basic?
Answer:
We can help the farmers by testing the soil in their fields.
Activities and Projects
1. Prepare different greeting cards by using indicators.
Answer:
I had prepared a greeting card using turmeric indicator.
I took a white card board and cut it in the shape of a greeting card.
Applied turmeric paste on it and allowed to dry.
I draw designs and wrote wishes on it with soap water using a point brush.
It turns in to reddish brown.
Thus, I prepared greeting card using indicator.
Question 2.
Prepare beetroot indicator and test some acids and bases. Write a report.
Answer:
I have crushed beetroot and collected its juice.
This juice is filtered and used as an acid-base indicator.
When acids are added to this indicator there is no change in colour.
But when bases are added to this, it changed its colour from dark red to yellow. With this I concluded that the beetroot indicator is used to test the bases.
Test with beetroot indicator
| Solution | Colour changes into yellow |
| 1. Lemon juice | ✗ |
| 2. Glass cleaner | ✓ |
| 3. Water | ✗ |
| 4. Sodium hydroxide | ✓ |
| 5. Potassium hydroxide | ✓ |
| 6. Hydrochloric acid | ✗ |
Question 3.
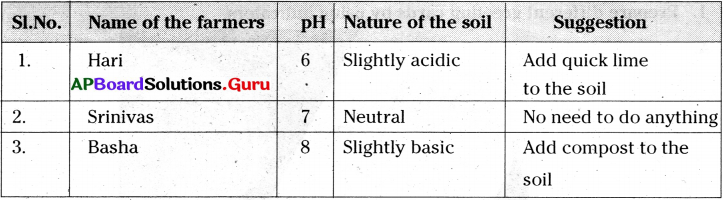
Visit various agricultural fields and collect soils samples. Do the soil test. Make a report.
Answer:
I have visit nearest agricultural fields and collected soil from the fields. I took 10 g of soil from each sample in a beaker and added 500 ml of water to it. I stirred it well and filtered the solution. Then tested the filtered solution with a pH paper and tabulated the results. I gave suggestions to the farmers to neutralize their soils basing on the results.
Activities
Activity – 1
Question 1.
Taste some substances in our house and identify their nature through taste from your previous experiences. Fill the findings in the table.
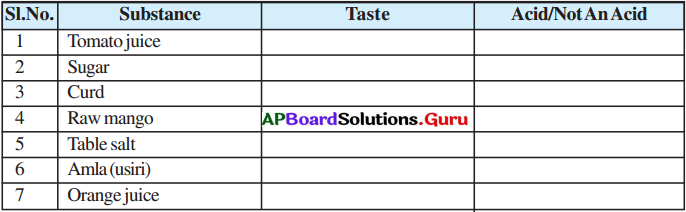
Answer:
| Substance | Taste | Acid/Not An Acid |
| 1. Tomato juice | sour | acid |
| 2. Sugar | sweet | not an acid |
| 3. Curd | sour | acid |
| 4. Raw mango | sour | acid |
| 5. Table salt | salt | not an acid |
| 6. Amla (usiri) | sour | acid |
| 7. Orange juice | sour | acid |
→ Which substances have sour taste in the above table? Why?
Answer:
Tomato juice, curd, raw mango, amla and orange juice have sour taste, because they contain acids. . .
Activity – 2
Question 2.
Take a soap into your palm and add some water. Now rub with another palm.
→ How do you feel while rubbing?
Answer:
You can feel the slippery nature of the soap.
Repeat this activity with tooth paste now. Observe your feeling.
Tooth paste is also slippery in nature.
These substances contain slippery chemicals they are called Bases. Bases are slip¬pery in nature and also bitter to taste.
Activity – 3
Question 3.
Take a table spoon of turmeric powder in a plate. Add little water and make it into a paste. Take a white paper and apply the paste over it on both sides and let it dry. After drying, cut the paper into strips. Now turmeric paper strips are ready for use as indicator.
→ What is the colour of these strips?
Answer:
They are yellow in colour.
Testing with turmeric indicator
- Take soap solution in a plate. Dip one turmeric paper strip in the soap solution.
- Take it out and observe the colour of the strip.
- Repeat the activity with lime water and lemon juice.
→ Do you observe any change in colour of tumeric strips?
Note your observations in the table.
Answer:
| Substance | Observed colour change |
| Soap solution | Reddish brown |
| Lime solution | Reddish brown |
| Lemon juice | No change |
The colour of turmeric strip will change into reddish brown in soap water and lime water, but its colour will not change in lemon juice.
Test with hibiscus indicator :
Take lemon juice, soda water, lime water, glucose solution, sugar solution, soap water etc.into test tubes. Add sufficient amount of hibiscus indicator in each test tube.Observe the change in the colour of the substance and record in the table.
| Name of the Substance | Observed colour change |
| 1. Lemon juice | Changed to pink |
| 2. Soda water | Changed to pink |
| 3. Lime water | Changed to green . |
| 4. Glucose solution | No Change |
| 5. Sugar solution | No Change |
| 6. Soap water | Changed to green |
7th Class Science Textbook Page No. 24
Question 4.
How do you prove that hibiscus could be used as an acid base indicator?
(OR)
How do you prepare a hibiscus indicator?
Answer:
- Take some hibiscus (china rose or mandara) petals.
- Take warm water into a beaker and place the petals in the beaker.
- Keep it until the water has completely turned into violet colour.
- Filter the solution with a strainer.
- Now hibiscus indicator is ready to test.
Test with indicator:
- Take lemon juice, soda water, lime water, glucose solution, sugar solution, soap water etc. into test tubes.
- Add sufficient amount of hibiscus indicator in each test tube.
- Observe the change in the colour of the substance and record in the table.
| Name of the substances | Observed colour change |
| 1. Lemon juice | pink |
| 2. Soda water | pink |
| 3. Lime water | green |
| 4. Glucose solution | no change |
| 5. Sugar solution | no change |
| 6. Soap water | green |
Conclusion :
In acids and bases the hibiscus indicator changes its colour. Hence, it can used as an acid base indicator.
![]()
Lab Activity – 1
Question 5.
Test the given solution with different indicators and prepare a report.
(OR)
How do you test different indicators change their colours in different solutions?
Answer:
1) Take the solutions a) Dilute hydrochloric acid b) Sodium hydroxide c) Acetic acid d) Salt solution e) Sugar solution f) Soap solution in test tubes.
2) Test them with a) Red litmus b) Blue litmus c) Methyl orange d) Phenolphthalein indicators.
Table
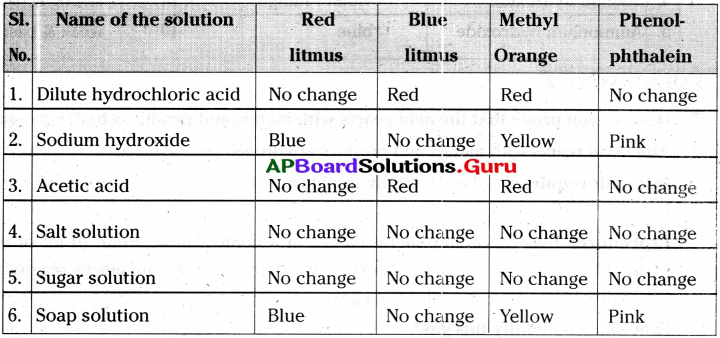
3) Blue litmus turns red in acids.
4) Red litmus turns blue in bases.
5) Methyl orange turns red in acids and yellow in bases.
6) Phenolphthalein turns pink in bases, but does not change its colour in acids.
7) In neutral substances all the above indicators do not change their colour.
Activity – 4
Question 6.
How do you find the strength and nature of the following substances?
1) Dilute hydrochloric acid
2) Vinegar
3) Water
4) Sodium hydroxide
5) Ammonium hydroxide.
Answer:
Take dilute hydrochloric acid, vinegar, water, sodium hydroxide and ammonium hydroxide in test tubes.;Now take universal indicator and add 2 drops in each test tube. You can observe that the solutions in the test tubes change into different colours. Now compare these colours with pH colour chart which was given on the indicator bottle.
Try to find pH values or the strengths Of acids and bases based on the colours they show and write them in the table.
Table
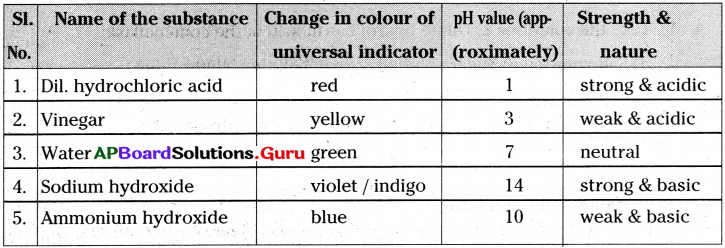
Lab Activity – 2
Question 7.
How do you prove that the acid reacts with metals and produces hydrogen gas?
Answer:
Aim: Acid Reacts with metals and produces hydrogen gas.
Materials required:
Conical flask, hydrochloric acid, zinc pieces, incense stick, match box.
Procedure:
Take 5g of zinc metal pieces in a conical flask. Pour 20 ml of dilute hydrochloric acid into it. Now, observe what happens. The zinc pieces reacted with the hydrochloric acid and releases a gas.
How can we identify this gas?
Test for hydrogen gas :
Now, introduce a incense stick into mouth of the conical flask. The flame of incense stick will put off with a pop sound. This is a test for hydrogen gas. Hydrochloric acid reacts with zinc metal and forms zinc chloride, releases hydrogen gas. This reaction can be written as a word equation,
Zinc+hydrochloric acid → zinc chloride+hydrogen
Conclusion:
Hence, we can conclude that acids react with metals and release hydrogen gas.
Activity – 5
Question 8.
How do you prove that bases react with metal and releases hydrogen gas ?
Answer:
Aim: Base reacts with metal and produces hydrogen gas.
Material required :
Conical flask, sodium hydroxide solution, zinc pieces, incense stick.
Procedure:
- Take 5gr of zinc metal pieces in a conical flask,
- Pour 20 ml of sodium hydroxide solution in it.
- Heat the conical flask.
Observation :
The zinc pieces reacts with base and releases gas.
Test for hydrogen gas :
- Now, introduce a incense stick into the mouth of the conical flask.
- The flame of incense stick will put off with a pop sound.
- This is a test for hydrogen gas.
Conclusion :
Bases react with metals and produce hydrogen gas.
Note : All bases do not react with metals.
![]()
Activity – 6
Question 9.
How do you prove that the carbon dioxide gas releases when an egg shell reacts with an acid?
(OR)
What happens when egg shells are dipped in an acid? How do you prove it?
Answer:
Take some crushed egg shells in a test tube and pour dilute Hydrochloric acid until the egg shells completely sink. Egg shell is made of calcium carbonate. Bring a burning match stick near the mouth of test tube.
The gas that put off the burning match stick is carbon dioxide. It was released by the action of acid with calcium carbonate.
Activity – 7
Question 10.
How do you perform a neutralization reaction in your lab?
Answer:
- Take sodium hydroxide solution in a conical flask and observe its colour. Now add 2-3 drops of phenolphthalein indicator to it.
- Now observe the colour. It is pink in colour.
- Using a dropper add dilute hydrochloric acid drop by drop to this solution and stir gently.
- Continue adding of the acid, till the pink colour disappears.
- The solution now is no more basic as it was neutralized by acid.
- We know that the colour of phenolphthalein indicator is pink in bases and colourless in acids and neutral substance.
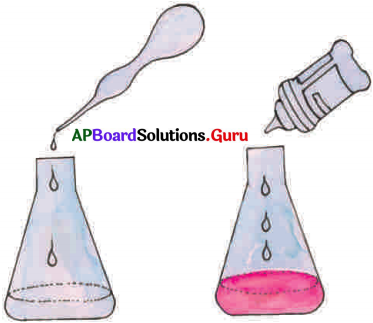
- The substances are salt and water because acids react with bases to form salts and water,
- Neutralization is a chemical reaction where acid and base reacts with each other to forms salt and water.
- Word equation of the above reaction is
Hydrochloric acid + sodium hydroxide → Sodium chloride + water - Now add one or more drops of hydrochloric acid to the solution. The pink colour disappears, as it has again become acidic.
- We can conclude that acids react with bases to produce salt and water.
Activity – 8
Question 11.
How do you test soil of agricultural field?
Answer:
Visit nearest agricultural fields and collect soil from the fields.
Take 10 g of soil in a beaker. Add 500 ml of water to it and stir well. Filter the solution. Now test the filtered solution with universal indicator or a pH paper and check your results.
| Field | pH | Nature of the soil |
| Field -1 | 6 | Slightly acidic |
| Field -2 | 7 | Neutral |
| Field -3 | 8 | Slightly basic |