Practice the AP 8th Class Physical Science Bits with Answers Chapter 3 Matter Around Us on a regular basis so that you can attempt exams with utmost confidence.
AP State Syllabus 8th Class Physical Science Bits 3rd Lesson Matter Around Us with Answers
Choose the correct answer.
Question 1.
Which of the following is more compressible ?
A) wood
B) air
C) water
D) sponge
Answer:
B) air
Question 2.
Powdered salt is a
A) solid
B) liquid
C) gas
D) none
Answer:
A) solid
![]()
Question 3.
Rate of diffusion is high in
A) ink drop
B) KMnO4 solution
C) oxygen
D) KMnO4 crystal
Answer:
C) oxygen
Question 4.
Which of the following is true in case of particles of matter ?
A) tiny
B) have space between them
C) have force of attraction between them
D) all the above
Answer:
D) all the above
Question 5.
The reason for high rate of diffusion in gas is
A) higher speed of gas particles
B) greater space between gas particles
C) both A and B
D) none
Answer:
C) both A and B
Question 6.
The melting point depends on
A) space among its particles
B) force of attraction among its particles
C) shape of the substance
D) state of the substance
Answer:
B) force of attraction among its particles
![]()
Question 7.
Evaporation is influenced by
A) surface area
B) humidity
C) wind speed
D) all
Answer:
D) all
Question 8.
Matter occupies ……………. and has …………….
A) length, mass
B) space, mass
C) mass, space
D) none of these
Answer:
B) space, mass
Question 9.
The state of matter which have a defi-nite shape and a fixed volume is …………
A) solids
B) liquids
C) gas
D) none of these
Answer:
A) solids
Question 10.
The state of matter which get the shape of container is ………..
A) solid
B) liquid
C) gas
D) plasma
Answer:
B) liquid
![]()
Question 11.
Liquids can flow easily from one place to another. So they are called …………
A) rigid substances
B) floaters
C) fluids
D) volatile
Answer:
C) fluids
Question 12.
………….. have no fixed shape, but have a fixed volume.
A) Solid
B) Liquid
C) Gases
D) Plasma
Answer:
B) Liquid
Question 13.
CNG stands for ………
A) Central Natural Gas
B) Central Nano Gas
C) Compressed Natural Gas
D) Conditional Natural Gas
Answer:
C) Compressed Natural Gas
Question 14.
…………. neither have a fixed shape nor volume.
A) Solids
B) Liquids
C) Gases
D) None of these
Answer:
C) Gases
![]()
Question 15.
Decrease in the volume of same quan-tity of gas under pressure is known as ……..
A) compressibility
B) rigidity
C) expansion
D) contraction
Answer:
A) compressibility
Question 16.
LPG stands for ………….
A) Litre Petroleum Gas
B) Longitudinal Petroleum Gas
C) Latitudinal Petroleum Gas
D) Liquefied Petroleum Gas
Answer:
D) Liquefied Petroleum Gas
Question 17.
…………. are highly compressible.
A) Liquids
B) Gases
C) Solids
D) Both A & B
Answer:
B) Gases
Question 18.
The movement of air, vapours of scent and smoke is known as ………..
A) diffusion
B) compressibility
C) rigidity
D) evaporation
Answer:
A) diffusion
![]()
Question 19.
The rate of diffusion is higher for ……..
A) solids
B) liquids
C) gases
D) none of these
Answer:
C) gases
Question 20.
All matter is made up of …….
A) very large particles
B) large particles
C) small particles
D) tiny particles
Answer:
D) tiny particles
Question 21.
Particles of matter have some …………. between them.
A) weight
B) mass
C) space
D) volume
Answer:
C) space
Question 22.
On dissolving solid in liquid, ………… particles enter into space between the …………. particles.
A) solid, liquid
B) liquid, liquid
C) liquid, solid
D) solid, solid
Answer:
A) solid, liquid
![]()
Question 23.
Particles of matter have ………….. between them that keeps the particles together.
A) force of repulsion
B) force of attraction
C) both
D) none of these
Answer:
B) force of attraction
Question 24.
Diffusion is possible only when the particles of matter …………
A) are stationary
B) move continuously
C) both
D) none of these
Answer:
B) move continuously
Question 25.
Space between the particles is very high in ………..
A) solid
B) liquid
C) gas
D) none of these
Answer:
C) gas
Question 26.
Rate of diffusion of gases is very high ………. because and ……….. of gas particles.
A) lower’speed, least space
B) higher speed, least space
C) higher speed, greater space
D) lower speed, greater space
Answer:
C) higher speed, greater space
![]()
Question 27.
Volume of water shinks between ……………
A) 0°C to 4°C
B) 50°C to 100°C
C) 60°C to 70°C
D) 100°C to 120°C
Answer:
A) 0°C to 4°C
Question 28.
The temperature at which solid melts to become a liquid is called ……..
A) boiling point
B) melting point
C) sublimation point
D) freezing point
Answer:
B) melting point
Question 29.
The process of melting is called …………..
A) evaporation
B) fusion
C) boiling
D) sublimation
Answer:
B) fusion
Question 30.
The melting point of substance depends ………….. on among the particles.
A) force of attraction
B) force to repulsion
C) both A & B
D) none of these
Answer:
A) force of attraction
![]()
Question 31.
As the force of attraction among the particles increases the melting point ………….
A) increases
B) decreases
C) does not change
D) none of these
Answer:
A) increases
Question 32.
The amount of heat energy that is required to overcome the attractive force among the particles to change the state is given by ………… of the substance.
A) specific heat
B) heat capacity
C) latent heat
D) none of these
Answer:
C) latent heat
Question 33.
The temperature at which liquid starts boiling at the atmospheric pressure is known as its ………….
A) boiling point
B) melting point
C) freezing point
D) sublimation point
Answer:
A) boiling point
Question 34.
Statement -1: Matter can change its state by changing temperature.
Statement – II: Matter can change its state by changing pressure.
A) Both statements -1 & II are true.
B) Statement -1 is true and statement – II is false.
C) Statement -1 is false and statement – II is true.
D) Both statements -1 & II are false.
Answer:
A) Both statements -1 & II are true.
![]()
Question 35.
300 K is equal to ……….. °C.
A) 37
B) 17
C) 27
D) 47
Answer:
C) 27
Question 36.
The phenomena of change of liquid into vapours at any temperature be-low its boiling point is called ………..
A) evaporation
B) sublimation
C) boiling
D) melting
Answer:
A) evaporation
Question 37.
As the surface area increases, the rate of evaporation ……………
A) increases
B) decreases
C) does not change
D) none of these
Answer:
A) increases
Question 38.
……………. is a surface phenomena.
A) Boiling
B) Melting
C) Evaporation
D) Sublimation
Answer:
C) Evaporation
![]()
Question 39.
…………….. is bulk phenomena.
A) Boiling
B) Evaporation
C) Condensation
D) None of these
Answer:
A) Boiling
Question 40.
Assertion (A): Salt crystal is not solid. Reason (R) : Shape of the salt crystals is depending on the shape of the container.
A) A’ and ‘R’ are true and ’R’ is a cor-rect explanation of A’
B) A’ and ‘R’ are true and ‘R’ is not a correct explanation of A’
C) A’ is false and ‘R’ is true
D) A’ and ‘R’ are false
Answer:
D) A’ and ‘R’ are false
Question 41.
Assertion (A) : Smell of petrol can identify from some distance. Reason (R) : Solids diffuse in liquid.
A) A and ‘R’ are true and ‘R’ is a cor-rect explanation of A’
B) A’ and ’R’ are true and ‘R’ is not a correct explanation of A’
C) A’ is false and ‘R’ is true
D) A’ and ‘R’ are false
Answer:
B) A’ and ’R’ are true and ‘R’ is not a correct explanation of A’
Question 42.
Which of the following statement is true ?
A. During respiration oxygen diffuses from lungs into blood.
B. During respiration carbon dioxide diffuses from blood to lungs.
A) A only
B) B only
C) A and B
D) None
Answer:
C) A and B
![]()
Question 43.
Who is correct ?
Sailaja : NH3 diffuses faster than HCl.
Lalitha: HCl diffuses faster than NH3.
A) Sailaja
B) Lalitha
C) Both
D) None
Answer:
A) Sailaja
Question 44.
Which of the following is a false state-ment?
A) There exists space between particles in gases.
B) Particles of matter attracts each other.
C) Particles of solids are bigger than that of gas particles.
D) None
Answer:
C) Particles of solids are bigger than that of gas particles.
Question 45.
Match the following:
1) Water – KMnO4 crystals — a) solid diffuses in liquid
2) Air – SO2 gas — b) gas diffuses in gas
3) Petrol – Kerosene — c) liquid diffuses in liquid
A) 1-a, 2-b, 3-c
B) 1-b, 2-a, 3-c
C) 1-a, 2-c, 3-b
D) 1-b, 2-c, 3-a
Answer:
A) 1-a, 2-b, 3-c
Question 46.
Which of the following statement is true ?
Poomima : Solids, liquids and gases diffuse into liquids.
Sheshu : Rate of diffusion of gases is higher than that of liquids or solids.
A) Poornima
B) Sheshu
C) A and B
D) None
Answer:
C) A and B
![]()
Question 47.
Assertion (A) : Rate of diffusion of gases is higher than that of liquids or solids.
Reason (RA): There is a greater space between gas particles in gases.
Reason (RB): Speed of gas particles in gas is more than that of liquids or solids.
Correct Reason to Assertion is / are
A) R1
B) R2
C) R1 and R2
D) Neither R1 nor R2
Answer:
C) R1 and R2
Question 48.
Assertion (A): Particles in water at 0°C have more energy as compared to particles in ice at the same temperature.
Reason (R): The particles in water release heat energy during the pro¬cess of conversion from ice to liquid.
A) A is true and R is false
B) A is false and R is true
C) Both A and R are true
D) Both A and R are false
Answer:
A) A is true and R is false
Question 49.
Which of the following statement is true ?
A) Chaitanya : Water can change into watervapour without reaching its boiling point.
B) Subbayya : Ice can change into water without reaching its melting point.
A) A
B) B
C) A and B
D) Neither A nor B
Answer:
A) A
Question 50.
Milk : Liquid :: Curd : …………..
A) Solid
B) Liquid
C) Gas
D) Plasma
Answer:
A) Solid
![]()
Question 51.
By the given conversation guess the substance ’A’.
Srilatha : Does ‘A’ possess fixed volume?
Susheela: Yes.
Srilatha : Does ‘A’ possess fixed shape?
Susheela: No.
A) Solid
B) Liquid
C) Gas
D) ‘B’ or ’C
Answer:
B) Liquid
Question 52.
Guess the shape of the water, if it spills on a floor is
A) circle
B) line
C) triangular
D) we cannot say
Answer:
D) we cannot say
Question 53.
One litre of water can fill in cylinder A and two litres of water can fill in cylinder B. Then two litres of gas can fill in
A) cylinder A
B) cylinder B
C) both ‘A’ and ‘B’
D) not possible
Answer:
C) both ‘A’ and ‘B’
Question 54.
State of the water at 274 K is
A) liquid
B) solid
C) vapour
D) we cannot say
Answer:
A) liquid
![]()
Question 55.
In a cloudy day wet clothes do not dry quickly – because
A) more surface area
B) high wind speed
C) high humidity
D) above all
Answer:
C) high humidity
Question 56.
The force of attraction between the particles of ………….. is strong.
A) liquid
B) gas
C) solid
D) none
Answer:
C) solid
Question 57.
The process of conversion of solid into liquid is
A) fusion
B) diffusion
C) boiling
D) changing
Answer:
A) fusion
Question 58.
Oxygen and Carbon dioxide from atmosphere diffuse and dissolve in wa-ter is essential for survival of ………..
A) human beings
B) land animals
C) birds
D) aquatic animals
Answer:
D) aquatic animals
![]()
Question 59.
Among ammonia and hydrogen chloride which gas travels faster?
A) Ammonia
B) Hydrogen chloride
C) Both travel with same speed
D) None of these
Answer:
A) Ammonia
Question 60.
Hydrochloric acid and Ammonia react together and form a white substance called ………
A) Ammonium hydride
B) Ammonium hydroxide
C) Ammonium chloride
D) Nitric acid
Answer:
C) Ammonium chloride
Question 61.
When we heat a substance the particles gain additional ………….
A) density
B) mass
C) energy
D) none of these
Answer:
C) energy
Question 62.
Particles of water at 0°C have …………. energy than the particles in ice at the same temperature.
A) more
B) less
C) same
D) none of these
Answer:
A) more
![]()
Question 63.
Generally, the given instrument is not suitable to measure solids.
A) Simple balance
B) Measuring jar
C) Spring balance
D) None
Answer:
B) Measuring jar
Question 64.
Take water into a syringe, close the hole at the nosil and push the piston. By this experiment you can conclude that
A) Liquids are compressible
B) Liquids are not compressible
C) Liquids contain space between their particles
D) Liquids do not contain space between their particles
Answer:
B) Liquids are not compressible
Question 65.
Take some ice cubes in a beaker. Insert a thermometer. Heat the beaker continuously upto changed into water. The reading in thermometer
A) Increases continuously
B) Decreases continuously
C) First increases then decreases
D) Increases then remains constant
Answer:
D) Increases then remains constant
![]()
Question 66.
These substances are required to prove that the speed of diffusion is different for different substances.
A) Test tube, potassium permanganate, water
B) Flask, copper sulphate, water
C) Long glass tube, cotton, HCl, NH3
D) Long glass tube, CuSO4 Sol., ZnSO4 Sol.
Answer:
C) Long glass tube, cotton, HCl, NH3
Question 67.
To prove the effect of surface area on evaporation we need
A) water, test tube, conical flask
B) water, test tube, China dish
C) water, China dish, saucer
D) water, petrol, test tubes
Answer:
B) water, test tube, China dish
Question 68.
What do you to change a substance from gas state to liquid state ?
A) By giving heat
B) By cooling
C) By increasing pressure
D) By decreasing pressure
A) A or C
B) B or C
C) A or D
D) B or D
Answer:
D) B or D
Question 69.
The gas that diffuses from lungs into blood is ………….
A) oxygen
B) carbon dioxide
C) water vapour
D) hydrogen
Answer:
A) oxygen
![]()
Question 70.
The gas that diffuses from blood into lungs is …………
A) oxygen
B) carbon dioxide
C) water vapour
D) hydrogen
Answer:
B) carbon dioxide
Question 71.
Boiling point of water ……….
A) 0°C
B) 100°C
C) 373 K
D) Both B & C
Answer:
D) Both B & C
Question 72.
Solid carbon dioxide is known as …………
A) wet ice
B) dry ice
C) ice
D) liquid ice
Answer:
B) dry ice
Question 73.
The amount of water vapour present in air is called ………..
A) pressure
B) sweating
C) humidity
D) evaporation
Answer:
C) humidity
![]()
Question 74.
As the humidity increases, the rate of evaporation ………….
A) increases
B) decreases
C) does not change
D) none of these
Answer:
B) decreases
Question 75.
As the wind speed increases, the rate of evaporation …………
A) decreases
B) increases
C) does not change
D) none of these
Answer:
B) increases
Question 76.
Water, milk, oil, stone, wood, rain-bow, book, clouds, smoke.
Which of the above is odd one ?
A) clouds
B) rainbow
C) water
D) smoke
Answer:
B) rainbow
Question 77.
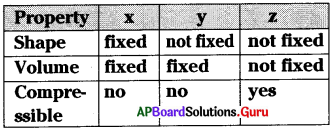
From the above table, x, y and z are ……………. respectively.
A) solid, liquid, gas
B) solid, gas, liquid
C) liquid, solid, gas
D) liquid, gas, solid
Answer:
A) solid, liquid, gas
![]()
Question 78.
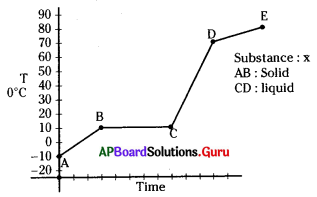
The boiling and melting points of the given substance ‘X’ are
A) 10°C, 80°C
B) 80°C, 10°C
C) 80°C, – 10°C
D) -10°C, 80°C
Answer:
B) 80°C, 10°C
Question 79.
A is liquid
B is solid
C is gas
Which of the above has both fixed shape and volume?
A) A
B) B
C) C
D) A and B
Answer:
B) B
Question 80.
H2O at 0°C – State (A)
H20 at 100°C – State (B)
H20 at 80°C – State (C)
Which state is solid state ?
A) State (A)
B) State (B)
C) State (C)
D) None
Answer:
D) None
![]()
Question 81.
(1) Wood, (2) Water, (3) Kerosene → Set 1
Which of the above evaporates quickly ?
A) Cl)
B) (2)
C) (3)
D) (2) and (3)
Answer:
C) (3)
Question 82.
In the given figure, identify the name of the material used in the dropper.

A) Blue ink
B) Red ink
C) Potassium permanganate
D) All
Answer:
D) All
Question 83.
What information does the given figure shows?
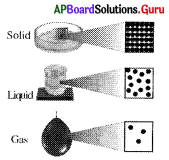
A) Arrangement of particles in solids
B) Arrangement of particles in liquids
C) Arrangement of particles in gases
D) All the above
Answer:
D) All the above
![]()
Question 84.
Which type of activity does the given figure represents?
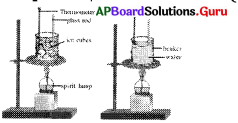
A) Effect of temperature on change of state
B) Changes of matter
C) Diffusion of two gases
D) None
Answer:
A) Effect of temperature on change of state
Question 85.

Label the parts a and b as …………, ………….. is correct.
A) HCl, NH3
B) NH3, HCl
C) HCl, Cl2
D) Cl2, HCl
Answer:
B) NH3, HCl
Question 86.
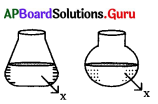
‘x’ is a substance with same volume. Hence ‘x’ can be labelled as
A) liquid
B) gas
C) A or B
D) solid
Answer:
A) liquid
![]()
Question 87.
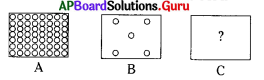
If you draw figure ’C’, how do you draw?
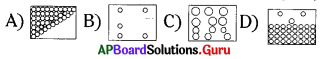
Answer:
C)
Question 88.
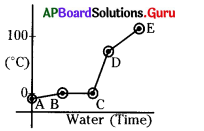
Identify the solid state.
A) AB
B) BC
C) CD
D) DE
Answer:
A) AB
Question 89.
Sweating gives cooling while
A) condensing
B) evaporating
C) both A and B
D) boiling
Answer:
B) evaporating
Question 90.
LPG cylinders are very appreciable because,
A) LPG has no fixed shape
B) LPG has fixed volume
C) LPG is compressible
D) LPG is not compressible
Answer:
C) LPG is compressible
![]()
Question 91.
We store water in earthen pots in winter because,
A) pots contain tiny holes and evaporates water.
B) pots contain tiny holes and condensate water.
C) pots obsorb heat.
D) water absorb heat.
Answer:
A) pots contain tiny holes and evaporates water.
Question 92.
If humidity increases, then rate of evaporation of sweating over the body
A) increases
B) decreases
C) A or B
D) first increases and then decreases
Answer:
B) decreases
Question 93.
If the body temperature of a man is 34°C, it equals to
A) 34 K
B) 239 K
C) 234 K
D) 307 K
Answer:
D) 307 K
Question 94.
We prefer to sip hot tea with a saucer than a cup, because, saucer provides ………….. than a cup.
A) less volume
B) more volume
C) more surface area
D) less surface area
Answer:
C) more surface area
![]()
Question 95.
Steam produces more severe burns than boiled water, because steam contains energy of ……………. more than that of boiled water.
A) latent heat of fusion
B) latent heat of vapourisation
C) latent heat of melting
D) above all
Answer:
B) latent heat of vapourisation
Question 96.
We can observe water droplets on the outer surface of a glass containing ice cold water. Because
A) evaporation of ice – water in the glass.
B) evaporation of water vapour in the air.
C) condensation of ice cold water in the glass.
D) condensation of water vapour in the air.
Answer:
D) condensation of water vapour in the air.
Question 97.
Cotton clothes give comfort in summer. Because, they can support ……………. of the sweating.
A) condensation
B) evaporation
C) melting
D) above all
Answer:
B) evaporation
Question 98.
Rubber band is a
A) solid
B) liquid
C) gas
D) none
Answer:
A) solid
![]()
Question 99.
Sponge is a
A) solid
B) liquid
C) gas
D) none
Answer:
A) solid
Question 100.
Clothes can dry faster on
A) windy day
B) rainy day
C) sunny day
D) drier
Answer:
C) sunny day
Question 101.
Feeling cooler after sweating is an experience of
A) melting
B) boiling
C) sublimation
D) evaporation
Answer:
D) evaporation
Question 102.
For a given substance, in which state of matter is the space between its constituent particles the maximum?
A) In solid state
B) In liquid state
C) In gaseous state
D) Depends on the type of substance
Answer:
C) In gaseous state
![]()
Question 103.
During phase change the temperature of the substance
A) Keeps decreasing
B) Keeps increasing
C) Remains constant
D) First increases and then decreases
Answer:
C) Remains constant