Practice the AP 10th Class Physical Science Bits with Answers Chapter 7 Classification of Elements- The Periodic Table on a regular basis so that you can attempt exams with utmost confidence.
AP State Syllabus 10th Class Physical Science Bits 7th Lesson Classification of Elements- The Periodic Table with Answers
Question 1.
Number of elements discovered to till date.
A) 24
B) 63
C) 90
D) more than 115
Answer:
D) more than 115
Question 2.
……………. defined an element as any substance that cannot be decomposed into further simple substance by physical or chemical change.
A) Charles
B) Robert Boyle
C) Avagadro
D) Madam Curie
Answer:
B) Robert Boyle
Question 3.
Lithium, …………………… and Potassium constitute a Dobereiner triad
A) Magnesium
B) Calcium
C) Sodium
D) Rubidium
Answer:
C) Sodium
Question 4.
Louis Proust stated that……………….. atom is the building material.
A) Oxygen
B) Hydrogen
C) Chlorine
D) Nitrogen
Answer:
B) Hydrogen
Question 5.
Group of three elements with similar chemical properties is called …………….
A) diad
B) triad
C) tetrad
D) pentad
Answer:
B) triad
Question 6.
Dobereiner triad is based on
A) Atomic number
B) Atomic weight
C) Atomic volume
D) Atomic size
Answer:
B) Atomic weight
Question 7.
According to this every 8th element has similar properties starting from a, given element
A) Dobereiner triad
B) Newlands’ law
C) Mendeleeff’s periodic table
D) Modem periodic table
Answer:
B) Newlands’ law
![]()
Question 8.
Newlands’ periodic table was restricted to …………….. elements.
A) 40
B) 56
C) 60
D) 80
Answer:
B) 56
Question 9.
Dobereiner triad failed for …………………..
i) low mass
ii) equal mass
iv) neutral mass
A) Only i
B) both i and iii
C) both i and iv
D) all
Answer:
B) both i and iii
Question 10.
The number of periods and groups in Mendeleeff’s periodic table is
A) 7,8
B) 7,18
C) 10,18
D) 7,12
Answer:
A) 7,8
Question 11.
Mendeleeff’s periodic table is based on
A) atomic number
B) atomic weight
C) atomic volume
D) atomic size
Answer:
B) atomic weight
Question 12.
Match the following
a) eka boron — i) gallium
b) eka aluminium — ii) germanium
c) eka silicon — iii) scandium
A) a – i, b – ii, c – iii
B) a – iii, b – i, c – ii
C) a – iii, b – ii, c – i
D) a – i, b – iii, c – ii
Answer:
B) a – iii, b – i, c – ii
Question 13.
Atomic weight = ……………….. x equivalent weight
A) atomic number
B) combining capacity
C) valency
D) both B and C
Answer:
C) valency
Question 14.
Mendeleeff corrected atomic weights of
i) Beryllium
ii) Indium
iii) Gold
A) only i
B) only ii
C) both i and ii
D) all of these
Answer:
D) all of these
Question 15.
Element with atomic number 101 is
A) Barium
B) Fermium
C) Mendelevium
D) Einsteinium
Answer:
C) Mendelevium
Question 16.
Example for anomalous pair
A) Tellurium, Iodine
B) Cobalt, Nickel
C) Argon, Potassium
D) All of these
Answer:
D) All of these
Question 17.
The number of positive charges (protons) in the atom of an element is called………………
A) Atomic number
B) Mass number
C) Atomic weight
D) Atomic volume
Answer:
A) Atomic number
![]()
Question 18.
The atomic weight of a bivalent element is 9. The equivalent weight of same element is
A) 18
B) 13.5
C) 4.5
D) 3
Answer:
C) 4.5
Question 19.
Which pair of elements fits into same slot in Newlands’ table of elements ?
A) F, Cl
B) Co, Ni
C) Mg, Ca
D) C, Si
Answer:
B) Co, Ni
Question 20.
Law of octaves was proposed by
A) Dobereiner
B) Newlands
C) Neils Bohr
D) Mendeleeff
Answer:
B) Newlands
Question 21.
The predicted properties of elements Eka boron, Eka aluminium and eka silicon were close to the observed properties of the following elements respectively.
A) scandium, gallium, germanium
B) gallium, germanium, scandium
C) gallium, scandium, germanium
D) germanium, gallium, scandium
Answer:
A) scandium, gallium, germanium
Question 22.
The abnormal pair of elements in the following is
A) H and He
B) NeandAr
C) KrandK
D) K and Ar
Answer:
D) K and Ar
Question 23.
The set of elements that is not Dobereiner triad from the following
A) Ca, Sr, Ba
B) Cl ,Br, I
C) Mn, Cr, Fe
D) S, Si, Te
Answer:
D) S, Si, Te
Question 24.
Mendeleeff’s eka aluminium is
A) scandium
B) gallium
C) germanium
D) indium
Answer:
B) gallium
Question 25.
A Dobereiner triad in the following is
A) Cl, Br, I
B) H, He, Li
C) H, Na, Cl
D) C,N, O
Answer:
Answer:
A) Cl, Br, I
CONCEPT – 2 : Modern Periodic Table
Question 26.
……………….. found that each element emits a characteristic pattern of X ^ rays when subjected to bombardment by high energy electrons.
A) Bohr
B) Sommerfeld
C) Mendeleeff
D) Mosley
Answer:
D) Mosley
Question 27.
Modem periodic table is based on
A) atomic number
B) electron negativity
C) Both A and B
D) None of these
Answer:
A) atomic number
Question 28.
The number of periods and groups in modern periodic table ………………..
A) 7, 8
B) 7,18
C) 6,7
D) 5,6
Answer:
B) 7,18
Question 29.
Match the following
i) Sodium — a) p – block
ii) Aluminium — f -block
iii) Scandium — s – block
iv) Cerium — d) d-block
A) i → a, ii → b, iii → c, iv → d
B) i → a, ii → d, iii → b, iv →c
C) i → c, ii → a, iii → d, iv → b
D) i → d, ii → c,i ii → b, iv → a
Answer:
C) i → c, ii → a, iii → d, iv → b
Question 30.
Match the following
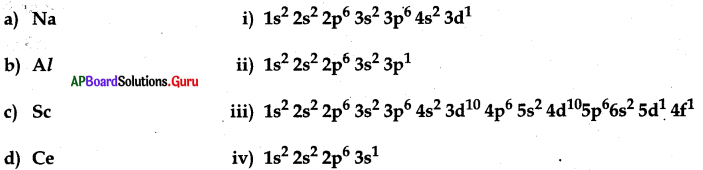
A) a → ii, b → i, c → iii, d → iv
B) a → iv, b →ii, c → i, d →iii
C) a → iv, b → iii, c → ii, d → i
D) a → iv, b → ii, c → iii, d → i
Answer:
B) a → iv, b →ii, c → i, d →iii
![]()
Question 31.
Match the following
a) Alkali metals i) VIA or 16
b) Alkaline earthmetals — i) I A or 1
c) Chalcogens — iii) VIIA or 17
d) Halogens — iv) II A or 2
A) a → i, b → ii, c iii, d →iv
B) a → iv, b → iii, c → ii, d → i
C) a → ii, b →iv, c → i, d → iii
D) a →iv, b → ii, c → iii, d → i
Answer:
C) a → ii, b →iv, c → i, d → iii
Question 32.
These are noble gases.
i) He
ii) Ne
iii) Ar
iv) Kr
A) i only
B) ii only
C) ii and iii
D) All of these
Answer:
D) All of these
Question 33.
…………….. elements are called Lanthanides.
A) 2f
B) 3f
C) 4f
D) 5f
Answer:
C) 4f
Question 34.
Lanthanides having atomic numbers from ……………… to ………………
A) 58, 71
B) 90,103
C) 60, 80
D) 90,110
Answer:
A) 58, 71
Question 35.
5 f elements are called ………………..
A) representative elements
B) transition elements
C) lanthanides
D) actinides
Answer:
D) actinides
Question 36.
Noble gases belong to ……………… group of periodic table.
A) IA
B) IIA
C) VIIA
D) VIIIA
Answer:
D) VIIIA
Question 37.
The elements present in 2nd period of long form of periodic table :
A) 2
B) 8
C) 18
D) 32
Answer:
B) 8
Question 38.
……………….. period of periodic table is incomplete.
A) 1st
B) 2nd
C) 6th
D) 7th
Answer:
D) 7th
Question 39.
The number of elements present in 6th period is ………………
A) 2
B) 8
C) 18
D) 32
Answer:
D) 32
Question 40.
Match the following
a) Shortest period — i) 7
b) Longest period — ii) 1
c) Incomplete period — iii) 5
d) Period having 18 elements — iv) 6
A) a → i, b → ii, c → iii, d →iv
B) a → iv, b →iii, c → ii, d → i
C) a →ii, b → iv, c → i, d → iii
D) a → iv, b → i, c → ii, d → iii
Answer:
C) a →ii, b → iv, c → i, d → iii
![]()
Question 41.
Nitrogen is the element of group-VA of the periodic table. Which of the following is the atomic number of the next element in the group?
A) 9
B) 14
C) 15
D) 17
Answer:
C) 15
Question 42.
Electron configuration of an atom is 2, 8, 7. Which of the following elements would it be chemically similar ?
A) Nitrogen (Z = 7)
B) Fluorine (Z = 9)
C) Phosphorous (Z = 15 )
D) Argon (Z = 18)
Answer:
B) Fluorine (Z = 9)
Question 43.
‘s’, ‘p’ block elements are collectively called as
A) Transition elements
B) Inner transition elements
C) Representative elements
D) Noble gases
Answer:
C) Representative elements
Question 44.
Statement I : d – block elements are called transition elements.
Statement II : f – block elements are called inner transition elements.
A) Both statements are true
B) Statement I is true and II is false
C) Statement I is false and II is true
D) Both statements are false
Answer:
A) Both statements are true
Question 45.
Inner transition elements belong to ………………
A) III B
B) IV B
C) V B
D) VI B
Answer:
A) III B
Question 46.
The element which is not metal in s – block is ………………..
A) Hydrogen
B) Chlorine
C) Sodium
D) Magnesium
Answer:
A) Hydrogen
Question 47.
Match the following.
a) valence of group IA — i) 4
b) valence of group IVA — ii) 0
c) valence of group VIA — iii) 1
d) valence of group VIIIA — iv) 2
A) a → i, b → ii, c → iii, d → iv
B) a → iv, b → iii, c → ii, d → i
C) a → iv, b → i, c → ii, d →iii
D) a → iii, b → i, c → iv, d → ii
Answer:
D) a → iii, b → i, c → iv, d → ii
Question 48.
Modern periodic law states that the properties of elements are periodic functions of their
A) atomic weight
B) atomic number
C) electron configuration
D) both B and C
Answer:
D) both B and C
Question 49.
Which of the following is the p block element ?
A) Ti
B) Ce
C) Ga
D) K
Answer:
C) Ga
![]()
Question 50.
How many s – block and p block elements are there in the third period of the periodic table ?
A) 2, 8
B) 8, 2
C) 4,4
D) 2, 6
Answer:
D) 2, 6
Question 51.
Metalloids are
A) s – block elements
B) d – block elements
C) f – block elements
D) p – block elements
Answer:
D) p – block elements
Question 52.
The ionization energy in the periodic table
A) decreases going down the group
B) increases going down the group
C) decreases from left to right in the period
D) remains same going down the group
Answer:
B) increases going down the group
Question 53.
As per modern periodic law, the properties of the elements are periodic functions of their
A) atomic weights
B) mass numbers
C) atomic numbers
D) valencies
Answer:
C) atomic numbers
Question 54.
Elements of which group are called halogens?
A) VA
B) VIA
C) VII A
D) IV A
Answer:
C) VII A
Question 55.
Which of the following elements belongs to d block ?
A) Cl
B) Cr
C) Sr
D) Ar
Answer:
B) Cr
Question 56.
The longest period in the modern periodic table is
A) 7th
B) 6th
C) 5th
D) 4th
Answer:
B) 6th
Question 57.
The valency of an element which belongs to 3rd period and 2nd group of periodic table is
A) 2
B) 3
C) 4
D) 5
Answer:
A) 2
Question 58.
The general electron configuration of inert gases
A) ns2
B) ns2np1
C) ns2np2
D) ns2np6
Answer:
D) ns2np6
Question 59.
The elements a, b, c, d have the following electron configurations. Then the elements that belong to the same group of periodic table is
a) 2,3
b) 2,8,3
c) 2,8,5
d) 2,8,7
A) a, b
B) b, c
C) c, d
D) d, a
Answer:
A) a, b
Question 60.
The inert gas element which does not have octet configuration in the outer
most orbit is
A) Ar
B) Kr
C) Rn
D) He
Answer:
D) He
Question 61.
The element belongs to 3rd period and 17th group is
A) F
B) Cl
C) Br
D) I
Answer:
B) Cl
Question 62.
The element with atomic number 13 belongs to ……………. period and …………….. group.
A) 3,13
B) 3, 2
C) 2, 3
D) 3,14
Answer:
A) 3,13
Question 63.
Where do Na and N belong ?
A) s-block
B) Na belongs to s – block and N belongs to d – block
C) p-block
D) Na belongs to s – block and N belongs to p – block
Answer:
D) Na belongs to s – block and N belongs to p – block
Question 64.
The atomic numbers of actinide series elements are
A) 58 to 71
B) 90 to 103
C) 92 to 105
D) 60 to 73
Answer:
B) 90 to 103
![]()
Question 65.
Which of the following group elements are known as chalcogens ?
A) 16
B) 6
C) 1
D) 17
Answer:
A) 16
Question 66.
The number of electrons that are present in Cl ion is
A) 6
B) 5
C) 11
D) 18
Answer:
D) 18
Question 67.
Which one of the following has the electronic configuration 1s2 2s2 2p6
A) Ne
B) Na+
C) F–
D) All
Answer:
D) All
Question 68.
Potassium and calcium belong to
A) s – block elements
B) p – block elements
C) d – block elements
D) f – block elements
Answer:
A) s – block elements
Question 69.
Which of the following are lanthan ides?
A) K to Kr
B) Cs to Lu
C) CetoLu
D) ThtoLr
Answer:
B) Cs to Lu
Question 70.
The element with highest electro – negativity belongs to
A) 3rd period and 17th group
B) 2nd period and 17th group
C) 2nd period and 16th group
D) 2nd period and 18th group
Answer:
B) 2nd period and 17th group
Question 71.
The general electron configuration of s – block elements are
A) ns1
B) ns2
C) ns1 to ns2
D) none of these
Answer:
C) ns1 to ns2
Question 72.
The general electron configuration of p – block elements are
A) ns1
B) ns32
C) ns2np1
D) ns2 np1 to ns2 np6
Answer:
D) ns2 np1 to ns2 np6
Question 73.
Cl belongs to …………… family.
A) Noble gases
B) Boron
C) Carbon
D) Halogen
Answer:
D) Halogen
Question 74.
Number of elements present in period 1 are
A) 2
B) 4
C) 6
D) 8
Answer:
A) 2
Question 75.
Which of the following element is electronegative?
A) Sodium
B) Oxygen
C) Magnesium
D) Calcium
Answer:
B) Oxygen
Question 76.
Number of vertical columns in the modern periodic table are
A) 7
B) 8
C) 10
D) 18
Answer:
D) 18
Question 77.
Choose the correct answer for the following matching.
Group A — Group B
1) Alkali metal — P) Calcium
2) Chalcogen — Q) Potassium
3) Alkaline earth metal — R) Sulphur
A) 1-Q,2-R, 3-P
B) 1-Q, 2-P, 3-R
C) 1-P, 2-0,3-R
D) 1 – P, 2 – R, 3 – Q
Answer:
A) 1-Q,2-R, 3-P
CONCEPT – 3 : Periodic Properties
Question 78.
The elements which have both metallic and non metallic properties are called
A) Metalloids
B) Semiconductors
C) Semi metals
D) Both A and C
Answer:
D) Both A and C
Question 79.
The examples for metalloids
A) Li, Na, K, Rb
B) Be, Mg, Ca, Sr, Ba
C) B, Si, As, Ge
D) F, Cl, Br, I
Answer:
C) B, Si, As, Ge
Question 80.
The distance from the centre of the nucleus of the atom to its outermost shell is
A) Atomic number
B) Atomic volume
C) Atomic radius
D) Atomic density
Answer:
C) Atomic radius
Question 81.
Atomic radius is measured in
A) m
B) mm
C) km
D) pm
Answer:
D) pm
Question 82.
1 pm = ……………. m
A) 10-3
B) 10-9
C) 10-12
D) 10-6
Answer:
C) 10-12
Question 83.
The correct ascending order of atomic size for the following elements C, Li, N, Be :
A) C, Li, N, Be
B) Li, Be, C, N
C) C, N, Be, Li
D) Be, Li, N, C
Answer:
B) Li, Be, C, N
![]()
Question 84.
Which among these have greater size?
A) Na
B) Na+
C) Mg2+
D) Al3+
Answer:
A) Na
Question 85.
Which of the following has greater size?
A) F
B) F
C) Cl
D) Cl–
Answer:
D) Cl–
Question 86.
Statement I: Positive ion of an element has less size than its neutral atom.
Statement II : Negative ion of an element has bigger size than the neutral atom.
A) Both statements are true
B) Statement I is true and II is false
C) Statement I is false and II is true
D) Both are false
Answer:
A) Both statements are true
Question 87.
The energy required to remove an electron from the outermost orbit or shell of a neutral gaseous atom is
A) atomic size
B) ionization energy
C) electron gain enthalpy
D) oxidation potential
Answer:
B) ionization energy
Question 88.
Choose the correct order of ionization energies.
A) I1 > I2 > I3
B) I3 > I2 > I1
C) I3 > I1 > I2
D) I1= I2 = I3
Answer:
B) I3 > I2 > I1
Question 89.
Which of the following statements is wrong ?
A) As screening effect increases ionization energy decreases
B) As atomic size increases ionization energy decreases
C) Ionization energy is expressed in KJ mol-1
D) Nitrogen has less ionization energy compared to oxygen because it has stable half filled configuration.
Answer:
D) Nitrogen has less ionization energy compared to oxygen because it has stable half filled configuration.
Question 90.
The energy liberated when an electron is added to neutral gaseous atom is
A) ionization energy
B) electron affinity
C) electron gain enthalpy
D) both B and C
Answer:
D) both B and C
Question 91.
Alkaline earth metals have electron gain enthalpy values as
A) negative
B) positive
C) zero
D) all of these
Answer:
B) positive
Question 92.
Statement I: Electron affinity of atom is negative.
Statement II : Electron affinity of uninegative ion is positive.
A) Both statements are true
B) Statement I is true and II is false
C) Statement I is false and II is true
D) Both are false
Answer:
A) Both statements are true
Question 93.
The tendency of bonded atom to attract the electron pair towards itself is……………
A) electro positivity
B) electronegativity
C) electron affinity
D) ionization energy
Answer:
B) electronegativity
Question 94.
……………. proposed electronegativity of an element as average value of ionization energy and electron affinity.
A) Milliken
B) Pauling
C) Sommerfeld
D) Planck
Answer:
A) Milliken
Question 95.
……………….. assigned the electronegativity values for elements on the basis of bond energies.
A) Milliken
B) Pauling
C) Maxwell
D) Planck
Answer:
B) Pauling
Question 96.
The most electronegative element and its electronegativity value is
A) Cl, 4.0
B) F, 2.0
C) F, 4.0
D) Br, 4.0
Answer:
C) F, 4.0
Question 97.
The tendency of an atom to lose electrons is …………..
A) Metallic character
B) Electronegative character
C) Electropositive character
D) Both A and C
Answer:
D) Both A and C
Question 98.
The tendency of an atom to gain electrons is ……………..
A) Non-metallic character
B) Electropositive character
C) Metallic character
D) Both B and C
Answer:
A) Non-metallic character
Question 99.
The element at the bottom of a group would be expected to show ……………… metallic character than the element at the top
A) less
B) more
C) same
D) can’t say
Answer:
B) more
Question 100.
Which of the following is the most active metal ?
A) Lithium
B) Sodium
C) Potassium
D) Rubidium
Answer:
D) Rubidium
Question 101.
Which of the following ions is larger in size ?
A) Na+
B) Mg2+
C) Al3+
D) H+
Answer:
A) Na+
Question 102.
Correct ionization energy order in following set of elements is ………………
A) C > O > N
B) N > O > C
C) O > N > C
D) N > C > O
Answer:
B) N > O > C
Question 103.
The element with negative charge having the electronic configuration 2,8, 8 is
A) O2-
B) O–
C) S2-
D) P–
Answer:
C) S2-
Question 104.
The pair of atomic numbers which belongs to the p-block elements is
A) 3,5
B) 11,12
C) 7,8
D) 12,13
Answer:
C) 7,8
![]()
Question 105.
Which one of the following decreases in a group from top to bottom ?
A) Atomic size
B) Metallic nature
C) Electropositivity
D) Electronegativity
Answer:
D) Electronegativity
Question 106.
The least electronegative element is
A) Cs
B) F
C) CZ
D) H
Answer:
A) Cs
Question 107.
Which of the following elements has larger atomic size ?
A) Na
B) Mg
C) Ca
D) K
Answer:
D) K
Question 108.
The correct order of electronegativity in the following elements is
A) F > Cl > O
B) F > O > Cl
C) O > F > Cl
D) Cl > F > O
Answer:
B) F > O > Cl
Question 109.
WZhich of the following elements has the highest electronegativity ?
A) CZ
B) F
C) Br
D) I
Answer:
B) F
Question 110.
As we go from left to right in a period the atomic number and atomic radius
A) both increase
B) both decrease
C) atomic number increases and atomic radius decreases
D) atomic number decreases and atomic radius increases
Answer:
C) atomic number increases and atomic radius decreases
Question 111.
Which of the following elements has more atomic radius
A) Be
B) Mg
C) Ca
D) Sr
Answer:
D) Sr
Question 112.
Which one of the following elements is electronegative ?
A) Na
B) O
C) Mg
D) Ca
Answer:
B) O
Question 113.
The biggest and smallest atoms from the following respectively are
A) N, Si
B) Si, N
C) C, N
D) N, P
Answer:
B) Si, N
Question 114.
The order of second ionization energy values of O and N is
A) O > N
B) N > O
C) 0 = N
D) IE2 is lessthan IE1
Answer:
B) N > O
Question 115.
Generally the order of electronegativity in group
A) decreases
B) increases
C) remains same
D) initially decreases then increases
Answer:
A) decreases
Question 116.
The correct order of atomic sizes of K, Ca, Na, Cl is
A) K > Ca > Na > CZ
B) K < Ca < Na < CZ C) Ca > K > CZ > Na
D) Na > CZ > K > Ca
Answer:
A) K > Ca > Na > CZ
![]()
Question 117.
Generally metallic character in period from left to right
A) increases
B) decreases
C) is equal for all elements
D) none
Answer:
B) decreases