Practice the AP 10th Class Physical Science Bits with Answers Chapter 6 Structure of Atom on a regular basis so that you can attempt exams with utmost confidence.
AP State Syllabus 10th Class Physical Science Bits 6th Lesson Structure of Atom with Answers
CONCEPT -I: Electromagnetic ctrum
Question 1.
Match the following:
a) Electron — ii) Negatively charged particle
c) Neutron — iii) Neutral particle
A) a → i, b → ii, c → iii
B) c → i, b → ii, a → iii
C) a → ii, b → i, c → iii
D) a → ii, b → iii, c → i
Answer:
C) a → ii, b → i, c → iii
![]()
Question 2.
The sub atomic particles present in an atom……………
i) electron
ii) proton
iii) neutron
A) only i
B) only ii
C) both i and ii
D) all of these
Answer:
D) all of these
Question 3.
The number of colours in rainbow
A) 3
B) 5
C) 7
D) 9
Answer:
C) 7
Question 4.
A vibrating charge produces ………………..
A) MechMhical wave
B) Stationary wave
C) Longitudinal wave
D) Electromagnetic wave
Answer:
D) Electromagnetic wave
Question 5.
Electromagnetic wave travels with a speed of …………….
A) 3 x 107 m/s
B) 3 x 106 m/s
C) 3 x 108 m/s
D) 3 x 109 m/s
Answer:
C) 3 x 108 m/s
Question 6.
Electromagnetic energy is charecterized by………………
i) wavelength
ii) amplitude
iii) frequency
A) only i
B) only ii
C) both i and iii
D) all of these
Answer:
C) both i and iii
Question 7.
The distance between two successive peaks of a wave is …………….
A) Amplitude
B) Frequency
C) Wavelength
D) Speed
Answer:
C) Wavelength
Question 8.
The relation between frequency and wavelength of electromagnetic wave
A) λ ∝ υ
B) λ = υ
C) λ ∝ 1/υ
D) none of these
Answer:
C) λ ∝ 1/υ
Question 9.
Formation of rainbow is example of …………….. spectrum.
A) UV
B) IR
C) RADIO
D) Visible
Answer:
D) Visible
![]()
Question 10.
V = υλ, is applicable for……………..
A) longitudinal waves
B) transverse waves
C) electromagnetic waves
D) all
Answer:
D) all
Question 11.
Red has …………………. wavelength and …………………. frequency.
A) maximum, maximum
B) minimum, maximum
C) maximum, minimum
D) minimum, minimum
Answer:
C) maximum, minimum
Question 12.
Quantum theory was proposed by……………….
A) Einsteiry
B) Maxwell
C) Max Planck
D) Newton
Answer:
C) Max Planck
Question 13.
Planck’s equation is …………..
A) E = hυ
B) E = h/υ
C) E = υ/h
D) None of these
Answer:
A) E = hυ
Question 14.
Which of the following are correct statements:
P: E = ko is called Planck’s equation
Q: ‘h’ is called Planck’s constant
R: ‘h’ value is 6.626 x 10-34J
S: According to Planck electro-magnetic energy is always emitted in multiples of h/υ
A) onlyP
B) PandQ
C) P, Q and R
D) All of these
Answer:
C) P, Q and R
Question 15.
When an iron rod is heated first it turns to
A) violet
B) red
C) green
D) orange
Answer:
B) red
Question 16.
Cupric chloride produces ………….. colour flame.
A) red
B) green
C) blue
D) orange
Answer:
B) green
Question 17.
Strontium chloride produces……………… colour flame.
A) red
B) green
C) blue
D) orange
Answer:
A) red
Question 18.
…………….. vapours produce yellow light in street lamps.
A) Potassium
B) Sodium
C) Calcium
D) Magnesium
Answer:
B) Sodium
Question 19.
Elements emit characteristic colours correspond to certain discrete wave¬lengths of light are called ……………..
A) bond spectra
B) absorption spectra
C) line spectra
D) both A and B
Answer:
C) line spectra
Question 20.
Line spectra is formed by ………………
A) atoms
B) molecules
C) ions
D) protons
Answer:
A) atoms
Question 21.
From the figure, the wavelength of Cosmic rays is………
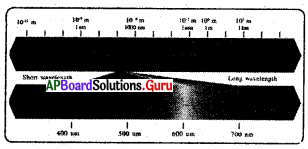
A) 10-3 m
B) 10-9 m
C) 103 m
D) 10-12 m
Answer:
D) 10-12 m
![]()
Question 22.
The wavelength range of. visible spectrum is……….
A) <400 nm B) >700 nm
C) 400 nm – 700 nm
D) 1mm-lm
Answer:
C) 400 nm – 700 nm
Question 23.
In this figure magnetic field and electric field are………. each other.
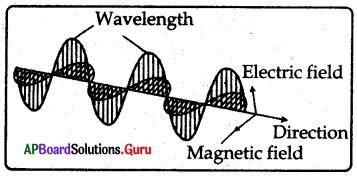
A) parallel to
B) perpendicular to
C) intersect
D) None of these
Answer:
B) perpendicular to
Question 24.
From the figure match the following:
a) X-rays — i) 700nm-1000nm
b) IRrays — ii) lm to 1 km
c) Radio rays — iii) Above 1 km
d) Broadcast — iv) 0.01 to 10 nm bond
A) a → iv, b → i, c → ii, d → iii
B) a →i, b → ii, c → iii, d → iv
C) a → iv, b → iii, c → ii, d → n
D) a → iii, b → iv, c →i, d → ii
Answer:
A) a → iv, b → i, c → ii, d → iii
Question 25.
…………. are used in Radar.
A) UVrays
B) IRrays
C) Microwaves
D) X-rays
Answer:
C) Microwaves
Question 26.
An emission spectrum consists of , bright spectral lines on a dark back ground. Which one of the following does not correspond to the bright j spectral lines ?
A) Frequency of emitted radiation
B) Wavelength of emitted radiation
C) Energy of emitted radiation
D) Velocity of light
Answer:
D) Velocity of light
Question 27.
The colour in the sequence VIBGYOR that has least wavelength is …………….
A) yellow
B) red
C) green
D) violet
Answer:
D) violet
Question 28.
Which electromagnetic waves are sensitive to our eyes ?
A) X – rays
B) Ultraviolet rays
C) Visible rays
D) Microwaves
Answer:
C) Visible rays
Question 29.
Rainbow is a …………….. spectrum.
A) continuous
B) discontinuous
C) visible
D) both A and C
Answer:
D) both A and C
CONCEPT – II :Atomic model Bohr’s model & Sommer feld’s model
Question 30.
Stationary orbits are proposed by
A) Rutherford
B) Thomson
C) Newton
D) Neils Bohr
Answer:
D) Neils Bohr
Question 31.
The energies of an electron in an orbit of atom can have only certain values. These orbits are called……………..
i) stationary orbits
ii) stationary states
iii) energy level,
A) only i
B) only ii
C) only iii
D) all of these
Answer:
D) all of these
Question 32.
The energy is quantized means ………………
A) Energy is fixed
B) Energy would increase
C) Energy would decrease
D) None of these
Answer:
A) Energy is fixed
![]()
Question 33.
Lowest energy state of an electron is known as ……..
A) excited states
B) ground state
C) fixed state
D) rest state
Answer:
B) ground state
Question 34.
Higher energy state of an electron is known as ………..
A) ground state
B) excited state
C) neutral state
D) fixed state
Answer:
B) excited state
Question 35.
The spectrum formed due to excited electron come back to ground state is ………………….
i) absorption spectrum
ii) emission spectrum
iii) fine spectrum
A) only i
B) only ii
C) both i and ii
D) all
Answer:
B) only ii
Question 36.
When electron absorbs energy from surroundings and moves to excited state the spectrum formed is ……………..
A) Emission spectrum
B) Absorption spectrum
C) Fine spectrum
D) Continuous spectrum
Answer:
B) Absorption spectrum
Question 37.
In the following statements which is not correct for Bohr’s model
A) It explains the spectrum of hydrogen
B) It unable to explain spectra of elements having more than one electron
C) It explains fine spectrum of hydrogen atom
D) Unable to explain Zeeman effect
Answer:
C) It explains fine spectrum of hydrogen atom
Question 38.
Elliptical orbits were introduced by ……………..
A) Neils Bohr
B) Sommerfeld
C) Planck
D) Maxwell
Answer:
B) Sommerfeld
Question 39.
The number of elliptical orbits present in Bohr’s third orbit
A) 1
B) 2
C) 3
D) 4
Answer:
B) 2
Question 40.
Quantum mechanical model was proposed by ………………..
A) Neils Bohr
B) Sommerfeld
C) Erwin Schrodinger
D) Lande
Answer:
C) Erwin Schrodinger
Question 41.
If n = 4 then the orbit is represented as ………………….
A) K
B) L
C) M
D) N
Answer:
D) N
Question 42.
The maximum number of electrons that can be occupied in a given shell is………………
A) n2
B) n
C) 2n
D) 2n2
Answer:
D) 2n2
Question 43.
The maximum number of electrons that can be accommodated in the L- shell of an atom is …………………
A) 2
B) 8
C) 18
D) 32
Answer:
B) 8
Question 44.
Maximum number of electrons M shell contain is …………………
A) 2
B) 8
C) 18
D) 32
Answer:
C) 18
Question 45.
Match the following:
a) n = 1 — i) L
b) n = 2 — ii) N
c) n = 3 — iii) K
d) n = 4 — iv) M
A) a → i, b → ii, c → iii, d → iv
B) a → iii, b→ i, c → iv, d → ii
C) a → iv, b → iii, c → ii, d→i
D) a → ii, b→ i, c → iii, d → iv
Answer:
B) a → iii, b→ i, c → iv, d → ii
![]()
Question 46.
Bohr’s model explain the line spectra of ………
A) H+ ion
B) Hatom
C) He atom
D) Li+ ion
Answer:
D) Li+ ion
Question 47.
If an element has 3 electrons in the M-shell then the element is …………………
A) Mg
B) Al
C) Si
D) Na
Answer:
B) Al
Question 48.
If number of electrons in M-shell is half of K-shell then atomic number and name of element.
A) 12, Magnesium
B) 11, Sodium
C) 13, Aluminium
D) 14, Silicon
Answer:
B) 11, Sodium
Question 49.
When an electron jumps from higher orbit to lower orbit in an atom the energy is …………….
A) absorbed
B) emitted
C) not changed
D) depends on atom
Answer:
B) emitted
CONCEPT – III: Quantum
Question 50.
The region of space around the nucleus where the probability of finding electron is maximum is called…………..
A) orbit
B) sub orbit
C) orbital
D) none of these
Answer:
C) orbital
Question 51.
The information given by principal quantum number……………….
i) size of the orbit
ii) energy of the orbit
iii) orientation of orbitals in space
iv) spin of electrons
A) only i
B) both i and ii
C) i, ii and iii
D) all of these
Answer:
B) both i and ii
Question 52.
For’n’the minimum value is . and the maximum value is….
A) 1, ∝
B) 0, n-1
C) -n, +n
D) 0, n+1
Answer:
A) 1, ∝
Question 53.
The quantum number which explains about size and energy of the orbit or shell is ……………
A) n
B) l
C) ml
D) ms
Answer:
A) n
Question 54.
For l the minimum value is ……………… and the maximum value is
A) 0, l
B) 0,n-1
C) -l, +l
D) -n, n
Answer:
B) 0,n-1
Question 55.
Match the following:
a) l = 0 — i) d – sub shell
b) 1 = 1 — ii) s – sub shell
c) 7 = 2 — iii) f – sub shell
d) 7 = 3 — iv) p – sub shell
A) a → i, b → ii, c → iii, d → iv
B) a → iv, b → mi, c → ii, d → i
C) a → ii, b → iv, c → i, d → iii
D) a → iii, b → i, c → iv, d → ii
Answer:
C) a → ii, b → iv, c → i, d → iii
![]()
Question 56.
For ml the minimum value is ………….. and the maximum value is………….
A) 0, n
B) 0, n-1
C) \(-\frac{1}{2},+\frac{1}{2}\)
D) -l , +l
Answer:
D) -l , +l
Question 57.
If l = 1 for an atom then the number of orbitals in its sub shell:
A) 1
B) 2
C) 3
D) 0
Answer:
C) 3
Question 58.
Number of orbitals in a sub shell is given by the formula
A) 2n2
B) n2
C) l + l
D) 2l + 1
Answer:
D) 2l + 1
Question 59.
Match the following:
Sub shell — Number of degenerate orbitals
a) s — i) 7
b) p — ii) 1
c) d — iii) 3
d) f — iv) 5
A) a→i, b→ii, c→iii, d→iv
B) a→iv, b→iii, c→ii, d→h
C) a—ii, b→iii, c→iv, d→i
D) a→iv, b→ii, o→iii, d→i
Answer:
C) a—ii, b→iii, c→iv, d→i
Question 60.
Match the following:
a) s – orbital — i) dumbell ,
b) p – orbital — ii) spherical
c) d – orbital — iii) double dumbell
A) a→i, b→ii, c→iii
B) a→iii, b→ii, c→h
C) a→ii, b→i, c→iii
D) a→ii, b→iii, c→i
Answer:
C) a→ii, b→i, c→iii
Question 61.
Statement I: Fdi p sub shell the ml values are-1,0,1
Statement II: For d sub shell the ml values are -2, -1,0,1, 2
A) Both statements are correct
B) Statement I is correct and II is incorrect
C) Statement I is. incorrect and II is correct
D) Both statements are incorrect
Answer:
A) Both statements are correct
Question 62.
a) s – sub shell i) Maximum number of electrons is 6
b) p – sub shell ii) Maximum number of electrons is 14
c) d – sub shell iii) Maximum number of electrons is 2
d) f – sub shell iv) Maximum number of electrons is 10
A) a→i, b→ii, c→iii, d→iv
B) a→iv, b→iii, c→ii, d→i
C) a→iii, b→i, c→iv, d→ii
D) a→iv, b→i, c→ii, d→iii
Answer:
C) a→iii, b→i, c→iv, d→ii
Question 63.
Find the odd one.
A) Principal quantum number – n
B) Angular momentum quantum number – l
C) Magnetic quantum number – ml
D) Spin quantum number – k
Answer:
D) Spin quantum number – k
Question 64.
The value of in ms for an electron spinning in clockwise direction is ………….. and for anti clockwise direction is …………..
A) \(-\frac{1}{2},+\frac{1}{2}\)
B) \(+\frac{1}{2},-\frac{1}{2}\)
C) 1,-1
D) -1,1
Answer:
B) \(+\frac{1}{2},-\frac{1}{2}\)
Question 65.
The clockwise spin in an orbital is represented by …………..
A) →
B) ←
C) ↑
D) ↓
Answer:
C) ↑
![]()
Question 66.
Anti clockwise spin of electron is represented by ……………
A) ↑
B) →
C) ←
D) ↓
Answer:
D) ↓
Question 67.
If two electrons have positive spin values,, then their spins are …………………..
A) perpendicular
B) intersected
C) parallel
D) anti parallel
Answer:
C) parallel
Question 68.
If two electrons have positive and negative spin values then their spins are ………………..
A) parallel
B) anti parallel
C) perpendicular
D) intersected
Answer:
B) anti parallel
Question 69.
During writing electronic configuration the electrons are distributed into
A) shells
B) sub shells
C) orbitals
D) all of these
Answer:
D) all of these
Question 70.
“No two electrons of same atom have same set of four quantum numbers”. This principle is known as ……………..
A) Hund’s principle
B) Aufbau principle
C) Pauli’s exclusion principle
D) Heisenberg uncertainty principle
Answer:
C) Pauli’s exclusion principle
Question 71.
Orbitals are filled with electrons in the order of increasing energy is given by ………….. principle.
A) Hund
B) Aufbau
C) Pauli’s exclusion
D) Heisenberg
Answer:
B) Aufbau
Question 72.
……………… means building up.
A) Quanta
B) Photon
C) Aufbau
D) Orbital
Answer:
C) Aufbau
Question 73.
Which of the following orbital has greater n + l value ?
A) 4p
B) 5s
C) 3d
D) 4f
Answer:
D) 4f
Question 74.
The n + l value of 3d orbital is ………………
A) 3
B) 4
C) 5
D) 6
Answer:
C) 5
Question 75.
The filling order of atomic orbitals is given by.
A) Bohr
B) Sommerfeld
C) Pauli
D) Moeller
Answer:
D) Moeller
Question 76.
The rule violated in this electron configuration is 1s2, 2s°, 2p3
A) Pauli’s exclusion principle
B) Hund’s rule
C) Aufbau principle
D) Heisenberg uncertainty principle
Answer:
C) Aufbau principle
![]()
Question 77.
The rule violated in the following electronic configuration is………….
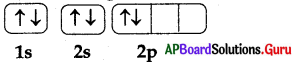
A) Pauli’s exclusion principle
B) Hund’s rule
C) Aufbau principle
D) Heisenburg uncertainty principle
Answer:
B) Hund’s rule
Question 78.
The principle is violated in the given information about Helium atom.
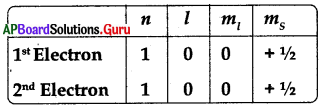
A) Pauli’s exclusion principle
B) Hund’s rule
C) Aufbau principle
D) Heisenberg uncertainty principle
Answer:
A) Pauli’s exclusion principle
Question 79.
According to Pauli’s exclusion principle the maximum number of electrons filled in an orbital is ………………
A) 1
B) 2
C) 3
D) 4
Answer:
B) 2
Question 80.
The valence electron configuration of electron for the following quantum numbers.
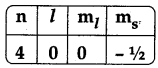
A) 4s1
B) 4s2
C) 4p2
D) 4p2
Answer:
B) 4s2
Question 81.
The valance electron configuration of electron for following quantum numbers.

A) 3d5
B) 3d6
C) 3d7
D) 3d10
Answer:
D) 3d10
Question 82.
The following orbitals have n+l value 6
i) 5p
ii) 6s
iii) 4d
iv) 4f
A) only i
B) both i and ii
C) i, ii and iii
D) all of these
Answer:
C) i, ii and iii
Question 83.
What are set of quantum numbers for electron configuration of element is 3d7?
A) n = 3, l = 2, ml = -1, ms = -1/2
B) n = 3, l =2, ml = -2, ms = -1/2
C) n = 3, l =2, ml= -1, ms = +1/2
D) n = 3, l = 2, ml= -2, ms = +1/2
Answer:
A) n = 3, l = 2, ml = -1, ms = -1/2
Question 84.
The quantum number which says about size and energy of orbit is
A) n
B) l
C) ml
D) ms
Answer:
A) n
Question 85.
Match the following:
a) Principal quantum number (n) — i) Orientation of orbital in space
b) Angular momentum quantum number (l) — Size and energy of orbit
c) Magnetic quantum number (ml) — iii) Shape of sub shell
d) Spin quantum number (ms )– Spin of electron
A) a→i, b→ii, c→iii, d→iv
B) a→ii, b→iii, c→i, d→iv
C) a→iv, b→iii, c→ii, d→i
D) a→iv, b→ii, c→iii, d→i
Answer:
B) a→ii, b→iii, c→i, d→iv
Question 86.
If n = 3 and Z = 2 the energy level is represented as
A) 3s
B) 3p
C) 3d
D) 3f
Answer:
C) 3d
![]()
Question 87.
To which boundary surface diagram of d – orbital does indicate ?
A) dxy
B) dyz
C) dz2
D) dx2-y2

Answer:
D) dx2-y2
Question 88.
The number of possible orbitals in a sub shell with angular momentum quantum number (1) is
A) l +1
B) 2l + 1
C) 2 (21 + 1)
D) 0 ton-1
Answer:
B) 2l + 1
Question 89.
The maximum number of electrons in n = 3 main energy level is ………………….
A) 8
B) 16
C) 18
D) 32
Answer:
C) 18
Question 90.
Which of the following quantum number describes the orientation of orbital in space around the nucleus of atom?
A) Magnetic quantum number
B) Spin quantum number
C) Angular momentum quantum number
D) Principal quantum number
Answer:
A) Magnetic quantum number
Question 91.
The correct electron configuration of Copper is……….
A) [Ar] 4s2 3d10
B) [Ar] 4s2 3d9
C) [Ar] 4s1 3d5
D) [Ar] 4s1 3d10
Answer:
D) [Ar] 4s1 3d10
Question 92.
The correct electron configuration of Chromium is………..
A) [Ar] 4s1 3d5
B) [Ar] 4s2 3d5
C) [Ar] 4s1 3d10
D) [Ar] 4s2 3d10
Answer:
A) [Ar] 4s1 3d5
Question 93.
The rule violated in electron configurations of Chromium and Copper is ………….
A) Aufbau
B) Hund
C) Pauli’s exclusion
D) Heisenberg uncertainty
Answer:
A) Aufbau
Question 94.
Atoms having these orbitals are stable
A) half filled
B) completely filled
C) both A and B
D) none of these
Answer:
C) both A and B
Question 95.
The maximum number of electrons accommodated in a sub shell with Azimuthal quantum number (angular momentum quantum number) l is ………………….
A) 2l + 1
B) 4l + 2
C) l(l+1)
D) 4l – 1
Answer:
B) 4l + 2
Question 96.
The four quantum numbers for valance electron of Sodium atom are ………………
A) n = 1, l = 0, ml= 0, ms = +1/2
B) n = 2, l = 0, ml= 0, ms = +1/2
C) n = 3, l = 0, ml= 0, ms = +1/2
D) n = 3, l = 1, ml= 0, ms= +1/2
Answer:
C) n = 3, l = 0, ml= 0, ms = +1/2
Question 97.
Degenerate orbitals have
A) same l value and same n value
B) different l value and same n value
C) same l value and different n value
D) same n+l value
Answer:
A) same l value and same n value
![]()
Question 98.
When l = 3 the maximum number of electrons that can occupy in the corresponding sub shell is ……………
A) 2
B) 6
C) 10
D) 14
Answer:
D) 14
Question 99.
The number of orbitals present in a given shell is …………
A) n
B) n2
C) 2n2
D) 2l + 1
Answer:
B) n2
Question 100.
The orbital that is having least energy among 3p, 4s, 3d, 4p is ……………………..
A) 3p
B) 4s
C) 3d
D) 4p
Answer:
A) 3p
Question 101.
Which of the following is the correct configuration of O2- …………….
A) 1s2 2s2 2p4
B) 1s2 2s2 2p6
C) 1s2 2s2 2p2
D) 1s2 2s2 2p5
Answer:
B) 1s2 2s2 2p6
Question 102.
The impossible set of quantum numbers for any electron of an atom is ……………………
a) n = 1, l = 1, ml = 0, ms = +1/2
b) n = 2, l =2, ml = 1, ms = -1/2
c) n = 3, l = 2, ml = 1, ms = +1/2
d) n = 3, l = 0, ml = 0, ms = -1/2
A) a only
B) a, b only
C) c, d only
D) d only
Answer:
B) a, b only
Question 103.
The orbitalwhich does not lie along the axis is………………
A) Px
B) d2 – y2
C) dxy
D) py
Answer:
C) dxy
Question 104.
Which one of the following has the electronic configuration of 1s2 2s2 2p6?
A) Ne
B) Na+
C) F–
D) All
Answer:
D) All
Question 105.
Magnetic quantum number of the last electron of Sodium is ………………….
A) 3
B) 2
C) 1
D) 0
Answer:
D) 0
![]()
Question 106.
The following is shape of …………… orbital.
A) s
B) p
C) d
D) f

Answer:
A) s