Practice the AP 0th Class Physical Science Bits with Answers Chapter 2 Acids, Bases and Salts on a regular basis so that you can attempt exams with utmost confidence.
AP State Syllabus 10th Class Physical Science Bits 2nd Lesson Acids, Bases and Salts with Answers
CONCEPT – I Properties Of Acids and Bases
Question 1.
Indicators are used to detect the nature of the solution for
A) Acidity
B) Basicity
C) Both A and B
D) None of these
Answer:
C) Both A and B
Question 2.
……………….. taste is a characteristic property of all acids in aqueous solutions
A) Sour
B) Bitter
C) Salty
D) Sweet
Answer:
A) Sour
![]()
Question 3.
Bases tend to taste …………….. and feel …………
A) sour, pleasant to touch
B) bitter, slippery to touch
C) sour, slippery to touch
D) None of these
Answer:
B) bitter, slippery to touch
Question 4.
Acids turn …………… litmus to ………………..litmus
A) red, red
B) blue, red
C) red, blue
D) blue, blue
Answer:
B) blue, red
Question 5.
Natural indicators are prepared from…..
i) plants
ii) petrochemicals
iii) living organisms
iv) minerals
A) only i
B) both i and ii
C) both i and iii
D) both ii and iv
Answer:
C) both i and iii
Question 6.
Natural indicators among these
i) litmus
ii) extract of red cabbage
iii) methyl orange
iv) turmeric
A) only i
B) both i and ii
C) i, ii and iv
D) All
Answer:
C) i, ii and iv
Question 7.
The colour of turmeric in basic solutions is………
A) blue
B) red
C) green
D) orange
Answer:
B) red
Question 8.
Synthetic indicator among these
A) Methyl red
B) Phenolphthalein
C) Methyl orange
D) All of these
Answer:
D) All of these
Question 9.
Match the following.
a) hydrochloric acid — i) H2SO4
b) sulphuric acid — ii) CH3COOH
c) nitric acid — iii) HCl
d) acetic acid — iv) HNO3
A) a → i, b→ii, c →iii, d → iv
B) a → iii, b →i, c →iv, d → ii
C) a →i, b →iii, c →iv, d → ii
D) a → i, b → iv, c →iii, d → ii
Answer:
B) a → iii, b →i, c →iv, d → ii
Question 10.
As aqueous acid solutions conduct electricity, they are identified as
A) electrolytes
B) bad conductors
C) good conductors
D) Both A and C
Answer:
D) Both A and C
Question 11.
Acids turn methyl orange into ……………
A) red
B) blue
C) yellow
D) pink
Answer:
A) red
![]()
Question 12.
Bases turn methyl orange into …………………
A) red
B) blue
C) yellow
D) pink
Answer:
C) yellow
Question 13.
Like acids, aqueous basic solutions conduct …………, and are identified as ………………
A) heat, electrolytes
B) electricity, electrolytes
C) electricity, nonelectrolytes
D) heat, partial electrolytes
Answer:
B) electricity, electrolytes
Question 14.
The colour of phenolphthalein indicator in basic solution is ………………….
A) yellow
B) green
C) pink
D) orange
Answer:
C) pink
Question 15.
Match the following.
a) Sodium hydroxide — i) Ca(OH)2
b) Calcium hydroxide — ii) NH4OH
c) Magnesium hydroxide — iii) Mg(OH)2
d) Ammonium hydroxide — iv) NaOH
A) a → i, b → ii, c → iii, d → iv
B) a → i,b → iii,c → ii,d → iv
C) a → iv, b → i, c →iii, d → ii
D) a → iv,b → iii,c → ii,d → i
Answer:
C) a → iv, b → i, c →iii, d → ii
Question 16.
Olfactory indicators are those indicators whose …….. change in acidic or basic media.
A) colour
B) odour
C) state
D) taste
Answer:
B) odour
Question 17.
The following will act as olfactory indicator.
A) onion
B) vanilla essence
C) clove oil
D) All of these
Answer:
D) All of these
Question 18.
The gas released in this experiment is.,
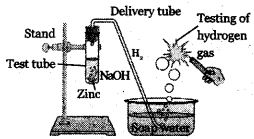
A) oxygen
B) hydrogen
C) chlorine
D) carbon dioxide
Answer:
B) hydrogen
Question 19.
More reactive metals react with acids and liberate …………….. gas.
A) O2
B) H2
C) Cl2
D) C02
Answer:
B) H2
Question 20.
Complete the reaction
2NaOH + Zn → ………………… + ………………………
A) NaCZ, H2
B) ZnO2, NaH2
C) Na2ZnO2, H2
D) Na2O, H2O
Answer:
C) Na2ZnO2, H2
Question 21.
Name the parts in the diagram.
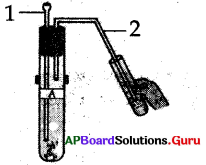
A) 1 – Thirstle funnel, 2 – Delivery tube
B) 1 – Cork, 2 – stand
C) 1 – Test tube, 2 – boiling tube
D) 1 – Sodium carbonate,
2 – hydrochloric acid
Answer:
A) 1 – Thirstle funnel, 2 – Delivery tube
Question 22.
Active metals react with acids and form
A) salt, hydrogen
B) salt, water
C) salt, nitrogen
D) salt, oxygen
Answer:
A) salt, hydrogen
Question 23.
A solution reacts with crushed egg shells to give a gas that turns lime water milky. The solution contains
A) NaCZ
B) HCZ
C) LiCZ
D) KCZ
Answer:
B) HCZ
Question 24.
What gas is produced when magnesium is made to react with hydrochloric acid?
A) Hydrogen
B) Oxygen
C) Carbon dioxide
D) No gas is produced
Answer:
A) Hydrogen
![]()
Question 25.
Metal oxides are generally …………… in nature
A) Acidic
B) Basic
C) Amphoteric
D) Neutral
Answer:
B) Basic
Question 26.
Non metal oxides are generally……. in nature
A) Acidic
B) Basic
C) Amphoteric
D) Neutral
Answer:
A) Acidic
Question 27.
Identify the odd one.
A) Glucose
B) Alcohol
C) Acetone
D) Hydrochloric acid
Answer:
D) Hydrochloric acid
Question 28.
………… is common to all adds.
A) H+(aq)
B) H3O+
C) OH–
D) Both A and B
Answer:
D) Both A and B
Question 29.
…….. does not change colour of dry blue litmus.
A) Hydrochloric acid
B) Sulphuric acid
C) Dry hydrogen chloride gas
D) Nitric acid
Answer:
C) Dry hydrogen chloride gas
Question 30.
……. is common for all bases.
A) H+(aq)
B) OH–(aq)
C) H3O+
D) OH+
Answer:
B) OH–(aq)
Question 31.
In water H+ ions exists as
A) H3O+
B) hydroniumion
C) OH–
D) both A and B
Answer:
D) both A and B
Question 32.
If a base dissolves in wafer by what name it is better known?
A) neutralization
B) basic
C) acid
D) alkali
Answer:
D) alkali
Question 33.
The process of dissolving an acid or base in water is ………….. process.
A) endothermic
B) exothermic
C) isothermal
D) adiabatic
Answer:
B) exothermic
Question 34.
Mixing of an acid or base with water result in decrease in the concentration of H3O+ /0H – per unit volume is called
A) hydration
B) dehydration
C) hydrolysis
D) dilution
Answer:
D) dilution
Question 35.
……………….. is slightly soluble in water
A) NaOH
B) KOH
C) Be(OH)2
D) None of these
Answer:
C) Be(OH)2
Question 36.
The nature of substance that converts blue litmus to red, but no change of colour with red litmus is
A) Base
B) Neutral
C) Acid
D) Can’t decide
Answer:
C) Acid
Question 37.
Phenolphthalein indicator gets pink colour with
A) HCl solution
B) NaOH solution
C) H2 SO4 solution
D) CH3 COOH solution
Answer:
B) NaOH solution
Question 38.
HCl solution with Na2 CO3 and NaHCO3 produces
A) H2 gas
B) NaH (sodium hydride)
C) H2 CO3
D) CO2 gas
Answer:
D) CO2 gas
Question 39.
Which of the following solutions convert red litmus paper to blue
A) HCl
B) HNO2
C) NaOH
D) None
Answer:
C) NaOH
Question 40.
Which of the following solutions give yellow colour with methyl orange indicator.
A) NaOH
B) CH3 COOH
c) Ha
D) H2 SO4
Answer:
A) NaOH
Question 41.
The formula of sodium zincate is
A) 2NaZnO
B) Na2 ZnO2
C) NaZnO2
D) NaZnO
Answer:
B) Na2 ZnO2
Question 42.
Olfactory indicator from the following is oaEaga
A) turmeric
B) onion
C) methyl orange
D) litmus paper
Answer:
B) onion
Question 43.
Pair of strong acid and strong base from the following is
A) HCl, NH4
B) CH3COOH, NH4OH
C) CH3COOH, NaOH
D) HCl, NaOH
Answer:
D) HCl, NaOH
Question 44.
Which one of the following produces more OH– ions ?
A) HCl solution
B) CH3COOH solution
C) NH4OH solution
D) NaOH solution
Answer:
D) NaOH solution
Question 45.
Which one of the following produces more number of H3O<sup+ ions
A) HCZ solution
B) CH3COOH solution
C) NaOH solution
D) Mg(OH)2 solution
Answer:
A) HCZ solution
Question 46.
Which of the following is a weak base?
A) KOH
B) NaOH
C) NH4OH
D) None of these
Answer:
C) NH4OH
![]()
Question 47.
When Zn is added to aqueous NaOH and on heating it forms
A) ZnO
B) Na2ZnO2
C) O2
D) Na2O
Answer:
B) Na2ZnO2
Question 48.
Which one of the following is an acid?
A) NaCZ
B) Ca(OH)2
C) HNO3
D) NaNO3
Answer:
C) HNO3
Question 49.
Which of the following indicators gives red colour-in acid solution ?
A) Methyl orange
B) Phenolphthalein
C) Litmus
D) Bromothymol blue
Answer:
A) Methyl orange
Question 50.
Which of the following is a mineral acid?
A) Oxalic add
B) Citric acid
C) Acetic acid
D) Phosphoric acid
Answer:
D) Phosphoric acid
Question 51.
A student added dil. HCZ to a test tube containing zinc granules and made the following observations.
i) The zinc surface becomes dull and black
ii) The gas evolved is burnt with a pop sound
iii) The solution remains colourless The correct observations are
A) i and ii
B) i and iii
C) ii and iii
D) i, ii and iii
Answer:
D) i, ii and iii
Question 52.
A student added few drops of universal indicator to a given colourless sample and observed the sample turning to red. The nature of sample is
A) neutral’solution
B) acid
C) base
D) either acid or base
Answer:
B) acid
CONCEPT – II Neutralization Reactions
Question 53.
When metal carbonate reacts with acid the products foiled are
A) salt
B) water
C) carbon dioxide
D) all of these
Answer:
D) all of these
Question 54.
Lime water turns into milky white due to the formation of
i) CaO
ii) Ca(OH)2
iii) CaCO3
iv) Ca(HCO3)2
A) only i
B) only ii
C) only iii
D) both ii and iii
Answer:
C) only iii
Question 55.
Statement I : When carbon dioxide passes through lime water it will turn into milky.
Statement II : When excess of carbon dioxide passes through lime water it will turn into colourless.
A) Both statements are true
B) Statement I is true and statement II is false
C) Statement I is false statement II is true
D) Both statements are false
Answer:
A) Both statements are true
Question 56.
The colourless solution formed when excess of CO2 is passed through slaked lime is
A) CaO
B) Ca(OH)2
C) CaCO3
D) Ca(HCO3)2
Answer:
D) Ca(HCO3)2
Question 57.
When acid reacts with base it forms salt and water then the reaction is called
A) Hydrolysis
B) Esterification
C) Neutralization
D) Dehydration
Answer:
C) Neutralization
Question 58.
Which of the following is the most accurate way of showing neutralization?
A) Acid + base → acid – base solution
B) Acid + base → salt + water
C) Acid + base → sodium chloride + hydrogen
D) Acid + base »neutral solution
Answer:
B) Acid + base → salt + water
Question 59.
Whichofthefollowingsubstanceswhen mixed together will produce table salt?
A) Sodium thiosulphate and sulphur dioxide
B) Hydrochloric acid and sodium hydroxide
C) Chlorine and oxygen
D) Nitric acid
Answer:
B) Hydrochloric acid and sodium hydroxide
Question 60.
Base reacts with…………….. to produce salt and
A) base, water
B) acid, water
C) salt,water
D) base, salt
Answer:
B) acid, water
Question 61.
Which one of the following types of medicines is used for treating irritation in stomach ?
A) antibiotic
B) analgesic
C) antacid
D) antiseptic
Answer:
C) antacid
Question 62.
The following are neutralization reactions.
i) Acid reacts with base to form salt and water
ii) Acid reacts with metal oxide to form salt and water
iii) Base reacts with non-metal oxide to form salt and water.
A) only i
B) both i and ii
C) both ii and iii
D) All of these
Answer:
D) All of these
Question 63.
Antacids are
A) strong acids
B) weak acids
C) strong bases
D) weak bases
Answer:
D) weak bases
Question 64.
The chemical name of milk of magnesia is…….
A) calcium hydroxide
B) magnesium hydroxide
C) potassium hydroxide
D) magnesium carbonate
Answer:
B) magnesium hydroxide
![]()
Question 65.
Family of chloride salts are
A) NaCl
B) KCl
C) MgCl2
D) All of these
Answer:
D) All of these
Question 66.
Milk of magnesia is
A) MgO
B) Mg(OH)2
C) MgCl2
D) Mg(HCO3)2
Answer:
B) Mg(OH)2
Question 67.
The pH of milk of magnesia is
A) 7 – 8
B) 6 – 7
C) 10 – 11
D) 4 – 5
Answer:
A) 7 – 8
Question 68.
HCl + H2O → X+ + Cl– Then X may be
A) H3O+
B) OH–
C) HOCl
D) H2O+
Answer:
A) H3O+
Question 69.
Which of the following bases is used in antacids ?
A) Ca(OH)2
B) NaOH
C) Mg(OH)2
D) NH4OH
Answer:
C) Mg(OH)2
Question 70.
…………….. is used for treating indigestion.
A) Antibiotic
B) Analgesic
C) Antacid
D) Antiseptic
Answer:
C) Antacid
Question 71.
Antacids are used
A) to produce acid in the stomach
B) to produce water in the stomach
C) to neutralise the excess base in the Stomach
D) to neutralize the excess acid in the stomach
Answer:
D) to neutralize the excess acid in the stomach
CONCEPT – III : pH and Its Applications
Question 72.
…………… can be used to know the strength of acid or base
A) Methyl orange
B) Phenolphthalein
C) Litmus
D) Universal indicators
Answer:
D) Universal indicators
Question 73.
The ‘p’ in pH stands for
A) potential
B) pressure
C) momentum
D) potenz
Answer:
D) potenz
Question 74.
Match the following.
a) acids — i) pH < 7 b) bases — ii) pH = 7 c) neutral — iii) pH > 7
A) a → i, b → iii, c →ii
B) a → i, b →ii, c → iii
C) a → iii, b → ii, c → i
D) a → ii, b →i, c → iii
Answer:
A) a → i, b → iii, c →ii
Question 75.
Match the following.
a) acid rain — i) pH below 5.5
b) tooth decay — ii) Milkof magnesia
c) antacid — iii) pH below 5.6
d) bee sting — iv) Methanoic acid
A) a → ii, b →i, c →iii, d → iv
B) a →iii; b → i, c →ii, d →iv
C) a →i, b → ii, c → iii, d → iv
D) a → i, b →iii, c →ii, d → iv
Answer:
B) a →iii; b → i, c →ii, d →iv
Question 76.
If the soil is acidic it is treated with……
A) Calcium hydroxide
B) Calcium carbonate
C) Acetic acid
D) A or B
Answer:
D) A or B
Question 77.
If the pH of a solution is 13.8 then it is
A) weak acid
B) weak base
C) strong acid
D) strong base
Answer:
D) strong base
Question 78.
Salt formed from a weak acid and strong base pH value is ……………..
A) < 7 B) > 7
C) 7
D) ≤ 7
Answer:
B) > 7
Question 79.
pH scale is introduced by
A) Sorensen
B) Mosley
C) Schrodinger
D) C.V. Raman
Answer:
A) Sorensen
Question 80.
A solution turned pink when a drop of phenolphthalein was added to it. The probable pH .of that solution is
A) 5
B) 6
C) 7
D) 10
Answer:
D) 10
Question 81.
Which of the following solutions has pH greater than 7 ?
A) CH3COOH
B) NH4Cl
C) NaCl
D) CH3COONa
Answer:
D) CH3COONa
Question 82.
Dissociation of ions in aqueous acetic acid is reversible process because it is a
A) weak acid
B) strong, acid
C) weak base
D) strong base
Answer:
A) weak acid
![]()
Question 83.
Tooth decay start when the pH value of mouth is
A) 5.4
B) 5.6
C) 5.7
D) 5.8
Answer:
A) 5.4
Question 84.
If a solution turns blue litmus to red then its pH is likely to be
A) 5
B) 8
C) 10
D) 12
Answer:
A) 5
Question 85.
Metal oxide + Acid → ……………..
A) salt + metal
B) salt + water
C) base + water
D) Non metallic oxide + base
Answer:
B) salt + water
CONCEPT – IV: Sortie Common Chemicals
Question 86.
The example for salt having acidic nature
A) NaCl
B) KCl
C) NH4Cl
D) CH3COONA
Answer:
C) NH4Cl
Question 87.
The example for salt having basic nature
A) NaCl
B) KCl
C) NH4Cl
D) CH3COONA
Answer:
D) CH3COONA
Question 88.
Brine solution among these is ……………….
A) NaCl(aq )
B) KCl(aq)
C) NH4Cl(aq )
D) MgCl2(aq )
Answer:
A) NaCl(aq )
Question 89.
The products formed in chlor-alkali process are
i) H2
ii) Cl2
iii) NaOH
iv) Na2CO3
A) only i
B) both i and ii
C) i, ii and iii
D) All of these
Answer:
C) i, ii and iii
Question 90.
The gases released at cathode and anode during chlor-alkali process
A) H2/Cl2
B) Cl2,H2
C) H2, O2
D) O2, H2
Answer:
A) H2/Cl2
Question 91.
Match the following
a) Bleaching powder i) NaHCO3
b) Baking soda ii) CaOCl2
c) Washing soda iii) HCOOH
d) Formic acid iv) Na2CO3.10H2O
A) a → i, b → ii, c → iii, d → iv
B) a → ii, b →i, c → iv, d → iii
C) a → i, b → iii, c → ii, d → iv
D) a → iv, b → iii, c → ii, d → i
Answer:
B) a → ii, b →i, c → iv, d → iii
Question 92.
……………. is used for disinfecting prinking water to make it free from germs.
A) Na2CO3.10H2O
B) NaHCO3
C) CaOCl2
D) Mg(OH)2
Answer:
C) CaOCl2
Question 93.
Baking powder is mixture of…………….and …………….
A) Baking soda, citric acid
B) Baking soda, tartaric acid
C) Washing soda, tartaric acid
D) Washing soda, citric acid
Answer:
B) Baking soda, tartaric acid
Question 94.
……………. is used in soda – acid extinguishers
i) Baking soda
ii) NaHCO3
iii) CaOCl2
iv) H2SO4
A) only i
B) Both i and ii
C) only iii
D) Both iii and iv
Answer:
B) Both i and ii
Question 95.
Washing soda is……………salt.
A) Acidic
B) Basic
C) Neutral
D) Amphoteric
Answer:
B) Basic
Question 96.
The water of crystallisation of hydrous copper sulphate is
A) 2
B) 5
C) 7
D) 9
Answer:
B) 5
![]()
Question 97.
The colour of hydrous copper sulphate is …………………
A) red
B) green
C) blue
D) orange
Answer:
C) blue
Question 98.
The colour of anhydrous copper sulphate is. …………..
A) white
B) blue
C) green
D) orange
Answer:
A) white
Question 99.
Match the following. .
a) Gypsum — i) CuSO4. 5H2O
b) Plaster of pans — ii) CaSO4. 2H2O
c) Hydrous copper sulphate — iii) CaSO4\(\frac { 1 }{ 2 }\)H2O
d) Anhydrous copper sulphate — iv) CuSO4
A) a → i, b →ii, c → iii, d → iv
B) a → iv, b → iii, c → ii, d → i
C) a → ii, b → iii, c → i, d → iv
D) a → i, b → iii, c → ii, d → iv
Answer:
C) a → ii, b → iii, c → i, d → iv
Question 100.
Which of the following is an antiseptic
A) Na2CO3
B) NaHCO3
C) NaCl
D) Na2SO4
Answer:
B) NaHCO3
Question 101.
Match the following.
i) Milk of magnesia — CaOCl2
ii) Gypsum — Mg(OH)2
iii) Bleaching powder — Na2CO3
iv) Washing soda — CaSO4. 2H2O
A) i → a, ii → c, iii → d, iv → b
B) i → d, ii → b, iii → a,iv → c
D) i → b, ii → a, iii → d, iv → c
C) i → b, ii → d, iii → a, iv → c
Answer:
D) i → b, ii → a, iii → d, iv → c
Question 102.
Which of the following salt solutions are basic in nature
A) NaiCl
B) NH4Cl
C) Na2CO3
D) KCl
Answer:
C) Na2CO3
Question 103.
Which of the following compound is used in glass, paper and soap industry?
A) Washing soda
B) Baking soda
C) Calcium hydroxide
D) Plaster of pads
Answer:
A) Washing soda
Question 104.
The formula of gypsum is
A) CaSO4.2H2O
B) CaSO4,3H2O
C) CaOCl2
D) CaSO4.\(\frac { 1 }{ 2 }\)H2O
Answer:
A) CaSO4.2H2O
Question 105.
The chemical formula of plaster of pans is
A) CuSO4.5H2O
B) CuSO4.H2O
C) CUSO4.\(\frac { 1 }{ 2 }\)H2O
D) CaSO4. \(\frac { 1 }{ 2 }\)H2O
Answer:
D) CaSO4. \(\frac { 1 }{ 2 }\)H2O
Question 106.
The difference of the water molecules between gypsum and plaster of paris is
A) \(\frac { 3 }{ 2 }\)
B) \(\frac { 1 }{ 2 }\)
C) 2
D) \(\frac { 5 }{ 2 }\)
Answer:
A) \(\frac { 3 }{ 2 }\)