Practice the AP 10th Class Physical Science Bits with Answers Chapter 11 Principles of Metallurgy on a regular basis so that you can attempt exams with utmost confidence.
AP State Syllabus 10th Class Physical Science Bits 11th Lesson Principles of Metallurgy with Answers
CONCEPT -I: Occurrence of the Metals & Reactivity of Metals
Question 1.
Metallurgy is .the process of extraction of ……………from the ores.
A) Non metals
B) Metals
C) Metalloids
D) Noble gases
Answer:
B) Metals
Question 2.
The alloy of copper and tin is ………………
A) Brass
B) Bronze
C) Steel
D) Stainless steel
Answer:
B) Bronze
![]()
Question 3.
The metals which are available in native form are ………………
A) Au
B) Ag
C) Cu
D) All of these
Answer:
D) All of these
Question 4.
The elements or compounds of the metals which occur in nature in the earth crust are called ………………
A) ores
B) minerals
C) gangue
D) slag
Answer:
B) minerals
Question 5.
The minerals from which the metals are extracted without economical loss are called ………………
A) gangue
B) slag
C) calcination
D) ore
Answer:
D) ore
Question 6.
The ore of aluminium is ………………
A) Clay
B) Bauxite
C) Horn silver
D) Haematite
Answer:
B) Bauxite
Question 7.
Match the following:
a) Horn silver — i) Kcl. MgCl2. 6H2O
b) Haematite — ii) AgCl
c) Cinnabar — iii) Fe2O3
d) Camalite — iv) Hgs
A) a → ii, b → iii, c→ iv, d → i
B) a→ i, b → ii, c → iii, d → iv
C) a → iv, b → iii, c → ii, d → i
D) a → iv, b → i, c → di, d → iii
Answer:
A) a → ii, b → iii, c→ iv, d → i
Question 8.
Arranging metals in the decreasing order of their reactivity is called ………….
A) Reactivity series
B) Lyman series
C) Balmer series
D) Pfund series
Answer:
A) Reactivity series
Question 9.
The following are examples for high reactivity mentals ………….
i) Potassium
ii) Sodium
iv) Magnesium
A) Only i
B) Both i and ii
C) i, ii and iii
D) All of these
Answer:
D) All of these
Question 10.
Ores of many metals are ………….
A) oxides
B) chlorides
C) sulphides
D) both A and C
Answer:
D) both A and C
Question 11.
Find the odd one out based on reactivity.
A) K
B) Na
C) Au
D) Ca
Answer:
C) Au
Question 12.
The ore of calcium metal among the following ………….
A) Bauxite
B) Lime stone
C) Rock salt
D) Haematite
Answer:
B) Lime stone
![]()
Question 13.
The fusible product formed when the impurity present in ore react with flux is called ………….
A) Gangue
B) Slag
C) Mineral
D) Alloy
Answer:
B) Slag
Question 14.
Carnalite is the ore of ………….
A) Pb
B) Mg
C) Hg
D) Zn
Answer:
B) Mg
Question 15.
The metal extracted from Gelena is ………….
A) Hg
B) Pb
C) Mg
D) Zn
Answer:
B) Pb
Question 16.
The metals having high reactivity from the following K, Na, Mg, Zn, Cu, Au
A) Zn, Cu, Au
B) Cu, Zn, Mg
C) K, Na, Mg
D) Na, Mg, Zn
Answer:
C) K, Na, Mg
Question 17.
The ore of mercury is ………….
A) Galena
B) Cinnabar
C.) Gypsum
D) Zincite
Answer:
B) Cinnabar
Question 18.
The metal which do not displace hydrogert from dil. HCl is ………………
A) Zn
B) Mg
C) Cu
D) Fe
Answer:
C) Cu
Question 19.
The formula of Gelena is
A) ZnS
B) MnO2
C) CaC03
D) PbS
Answer:
D) PbS
Question 20.
Match the following :
a) Copper Iron Pyrites — i) ZnS
b) Zinc blende — ii) Cu FeS2
c) Magnesite — iii) MgSO4. 7H2O
d) Epsom salt — iv) MgCO3
A) a → i, b → ii, c → iii, d → iv
B) a → iv, b → iii, c → ii, d → d
C) a → ii, b → d, c → iv, d → iii
D) a → iv, b → ii, c → i, d → iii
Answer:
C) a → ii, b → d, c → iv, d → iii
Question 21.
Match the following :
a) Horn silver — i) MnO2
b) Pyrolusite — ii) AgCl
c) Zincite — iii) NaCl
d) Rock salt — iv) ZnO
A) a → i, b→ii, c → iii, d →iv
B) a → iv, b → iii, c → ii, d → i
C) a → ii, b → i, c → iv, d → iii
D) a → iv, b → ii, c → i, d→iii
Answer:
C) a → ii, b → i, c → iv, d → iii
Question 22.
The reactivity of metals Al, Ag and Cu decrease in this order.
A) AZ > Cu > Ag
B) Ag > Cu > Al
C) Ag > Al> Cu
D) Cu > Ag > Al
Answer:
A) AZ > Cu > Ag
CONCEPT – II: Extraction of metals from their ores
Question 23.
The stages involved in extraction of metal from its ore ……………..
A) Concentration or dressing
B) Extraction of crude metal
C) Refining or purification of the metal
D) All of these
Answer:
D) All of these
Question 24.
The impurity present in ore is called……………..
A) Gangue
B) Flux
C) Slag
D) Mineral
Answer:
A) Gangue
![]()
Question 25.
Which of the following is a carbonate ore ?
A) Magnesite
B) Bauxite
C) Gypsum
D) Galena
Answer:
A) Magnesite
Question 26.
Which of the following is the correct formula of Gypsum ?
A) CUSO4.2H2O
B) CaSO4. 1/2 H2O
C) CuSO4. 5H2O
D) CaSO4. 2H2O
Answer:
D) CaSO4. 2H2O
Question 27.
Cinnabar is an ore of ……………..
A) Zn
B) Pb
C) Hg
D) Al
Answer:
C) Hg
Question 28.
The metal that occur in native form is……………..
A) Pb
B) Au
C) Fe
D) Hg
Answer:
B) Au
Question 29.
The most abundant metal in the earth’s crust is ……………..
A) silver
B) aluminium
C) zinc
D) iron
Answer:
B) aluminium
Question 30.
The oil used in the froth floatation process is……………..
A) kerosene oil
B) pine oil
C) coconut oil
D) olive oil
Answer:
B) pine oil
Question 31.
Froth floatation is a method used for the purification of ……………..ore.
A) sulphide
B) oxide
C) carbonate
D) nitrate
Answer:
A) sulphide
Question 32.
Getting rid of unwanted rocky material from ore is ……………..
A) Concentration
B) Enrichment of ore
C) Dressing
D) All of these
Answer:
D) All of these
Question 33.
The method used for concentration of Galena is ……………..
A) Hand picking
B) Froth floatation
C) Washing
D) Magnetic separation
Answer:
B) Froth floatation
Question 34.
The process used for concentration in the given diagram is ……………..
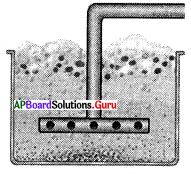
A) Hand picking
B) Washing
C) Froth floatation
D) Magnetic seperation
Answer:
C) Froth floatation
Question 35.
…………….. method is used for concentration of magnetite ore
A) Hand picking
B) Washing
C) Froth floatation
D) Magnetic seperation
Answer:
D) Magnetic seperation
Question 36.
The process involved in the given diagram is……………..
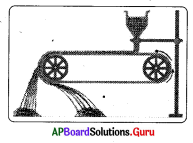
A) Hand picking
B) Washing
C) Froth floatation
D) Magnetic separation
Answer:
D) Magnetic separation
![]()
Question 37.
Normal reductions are not suitable for the above given activity series because……………..
A) Temperature required for reduction is too high
B) Temperature required for reduction is too small
C) More expensive
D) Both A and C
Answer:
D) Both A and C
Question 38.
The method used for extraction of metals at the top of the activity series is ……………..
A) Simple reduction of their oxides
B) Electrolysis of their aqueous solutions
C) Electrolysis of their fused compounds
D) All of these
Answer:
C) Electrolysis of their fused compounds
Question 39.
During electrolysis of NaCZ the substances formed at anode and cathode are ……………..
A) Na, Cl2
B) Cl2, Na
C) Na2, Cl
D) None of these
Answer:
B) Cl2, Na
Question 40.
The ores of metals in the middle of activity series generally present in the form of……………..
i) Oxides
ii) Chlorides
iii) Sulphates
iv) Sulphides
A) (i) and (ii)
B) (i) and (iii)
C) (ii) and (iii)
D) (i) and (iv)
Answer:
D) (i) and (iv)
Question 41.
In extraction of metals in the middle of activity series before reduction they are converted into ……………..
A) Metal chlorides
B) Mefal carbonates
C) Metal oxides
D) Metal sulphates
Answer:
C) Metal oxides
Question 42.
The ore is heated in the presence of oxygen or air below its melting point is called ……………..
A) Calcination
B) Roasting
C) Smelting
D) Poling
Answer:
B) Roasting
Question 43.
During Roasting …………….. ore is converted into …………….. ore.
A) Sulphide, Chloride
B) Chloride, Oxide
C) Sulphide, Oxide
D) Sulphide, Nitrate
Answer:
C) Sulphide, Oxide
Question 44.
The methods used to extract metals in the middle of the activity series are ……………..
i) Reduction of metal oxides with carbon
ii) Reduction of metal oxides with carbon mono oxide
iii) Auto reduction
iv) Reduction of ore by more reactive metals
A) Only (i)
B) Both (i) and (ii)
C) (i), (ii) and (iv)
D) All of these
Answer:
D) All of these
Question 45.
The reducing agent in thermite process is ……………..
A) Al
B) Mg
C) Fe
D) Si
Answer:
A) Al
Question 46.
When cinnabar is heated in air it first converted into …………….. and then reduced to ……………..
A) HgO, Hg
B) CuO, Cu
C) Hg, HgO
D) ZnO, Zn
Answer:
A) HgO, Hg
Question 47.
The process of obtaining the pure metal from impure metal is called ……………..
A) Roasting
B) Calcination
C) Smelting
D) Refining
Answer:
D) Refining
Question 48.
The …………….. method suitable for purification of low boiling metals.
A) Distillation
B) Poling
C) Liquation
D) Electrolytic refinery
Answer:
A) Distillation
Question 49.
The …………….. method is suitable for purification of low melting metals.
A) Distillation
B) Poling
C) Liquation
D) Electrolytic refinery
Answer:
C) Liquation
![]()
Question 50.
Blister copper is purified by using……………..
A) Distillation
B) Poling
C) Liquation
D) Calcination
Answer:
B) Poling
Question 51.
Match the following:
a) Distillation — i) Tin
b) Electrolytic refinery — ii) Zinc, Mercury
c) Liquation — iii) Copper
A) a→i, b → ii, c →iii
B) a → iii, b →ii, c → i
C) a →ii, b → iii, c → i
D) a →iii, b →i, c → ii
Answer:
C) a →ii, b → iii, c → i
Question 52.
The anode mud formed during electrolysis of acidified copper sulphate is ……………..
i) Antimony
ii) Selenium
iii) Tellurium
iv) Silver
A) Only (i)
B) Both (i) and (ii)
C) (i), (ii) and (iii)
D) All of these
Answer:
D) All of these
Question 53.
Tarnishing of silver is due to formation of ……………..
A) Silver chloride
B) Silver nitrate
C) Silver sulphate
D) Silver sulphide
Answer:
D) Silver sulphide
Question 54.
Corrosion of iron occurs in the presence of …………….. and……………..
A) kerosene, water
B) air, water
C) air, oil
D) kerosene, alcohol
Answer:
B) air, water
Question 55.
The chemical name of rust ……………..
A) Hydrated zinc oxide
B) Hydrated ferric oxide
C) Hydrated copper oxide
D) Hydrated calcium oxide
Answer:
B) Hydrated ferric oxide
Quesiton 56.
The formula of rust is……………..
A) Fe2O3
B) FeO
C) Fe2O3. XH2O.
D) Fe(OH)3
Answer:
C) Fe2O3. XH2O.
Question 57.
If carbon dioxide is dissolved in water it produces……………..acid
A) Carbonic
B) Hydrochloric
C) Chlorous
D) Sulphuric
Answer:
A) Carbonic
Question 58.
Pyro chemical process means process which takes place in the presence of ……………..
A) lightz
B) water
C) heat
D) electricity
Answer:
C) heat
Question 59.
Pyro chemical process among these :……………..
A) Smelting
B) Roasting
C) Calcination
D) All of these
Answer:
D) All of these
Question 60.
The purpose of smelting an ore is to …………….. it
A) oxidise
B) reduce
C) neutralise
D) none of these
Answer:
B) reduce
![]()
Question 61.
The chemical process in which the ore is heated in the absence of air is called ……………..
A) Roasting
B) Calcination
C) Smelting
D) All of these
Answer:
B) Calcination
Question 62.
The method used to convert magnesite to oxide ore is ……………..
A) Roasting
B) Calcination
C) Smelting
D) All of these
Answer:
B) Calcination
Question 63.
…………….. is substance added to ore to remove the gangue ?
A) Flux
B) Slag
C) Rust
D) Mineral
Answer:
A) Flux
Question 64.
Which of the following is useful to remove acidic gangue
i) CaO
ii) MgO
iii) FeO
iv) Si02
A) Only (i)
B) Both (i) and (ii)
C) (i), (ii) and (iii)
D) All of these
Answer:
C) (i), (ii) and (iii)
Question 65.
Which of the following is useful to remove basic gangue
A) SiO2
B) CO2
C) P2O5
D) All of these
Answer:
D) All of these
Question 66.
…………….. is used to carry pyro chemical process.
A) Furnace
B) Hearth
C) Chimney
D) Fire box
Answer:
A) Furnace
Question 67.
…………….. is place inside the funace where ore is kept for heating purpose.
A) Furnace
B) Hearth
C) Chimney
D) Fire box
Answer:
B) Hearth
Question 68.
For heamatite ore………………. is used as fuel and …………. is used as flux.
A) coke, quick lime
B) coke, lime stone
C) charcoal, lime stone
D) charcoal, quick lime
Answer:
B) coke, lime stone
Question 69.
Smelting is carried out in a…………….. furnace.
A) Reverberatory
B) Open hearth
C) Restart
D) Blast
Answer:
D) Blast
Question 70.
…………….. furnace is used for roasting.
A) Reverberatory
B) Open hearth
C) Retart
D) Blast
Answer:
A) Reverberatory
Question 71.
…………….. is the outlet through which flue (waste) gases go out of the furnace.
A) Hearth
B) clumney
C) Fire box
D) None of these
Answer:
B) clumney
Question 72.
…………….. is the part of furnace where the fuel is kept for burning.
A) Hearth
B) Chimney
C) Fire box
D) None of these
Answer:
C) Fire box
![]()
Question 73.
In……………..furnace both fire box and hearth are combined in big chamber which accommodates both ore and fuel.
A) Reverberatory
B) Open hearth
C) Retart
D) Blast
Answer:
D) Blast
Question 74.
…………….. furnace has both fire box and hearth separated.
A) Reverberatory
B) Open hearth
C) Retart
D) Blast
Answer:
A) Reverberatory
Question 75.
In……………..furnace there is no direct contact between the hearth and fire box.
A) Reverberatory
B) Open hearth
C) Retart
D) Blast
Answer:
C) Retart
Question 76.
Thermite reaction involves reaction of metal oxide with ……………..
A) Ag
B) Fe
C) Al
D) Hg
Answer:
C) Al
Question 77.
Generally metallic oxides are converted into metals by ……………..
A) Roasting
B) Calcination
C) Oxidation
D) Reduction
Answer:
D) Reduction
Question 78.
The reducing agent used to join railings of railway tracks is ……………..
A) Al
B) CO2
C) SO2
D) None of these
Answer:
A) Al
Question 79.
In electrolysis the reaction that takes place at cathode is ……………..
A) Oxidation
B) Reduction
C) Redox reaction
D) None
Answer:
B) Reduction
Question 80.
The impurities present in the metal are oxidized in this method ……………..
A) Liquation
B) Poling
C) Distillation
D) Auto reduction
Answer:
B) Poling
CONCEPT -III: Corrosion
Question 81.
Blast furnace is mainly suitable for ……………..
A) Smelting
B) Roasting
C) Calcination
D) Oxidation
Answer:
A) Smelting
Question 82.
The chemical formula of rust is
A) Fe2O3.XH2O
B) FeO
C) FeS
D) Fe2O5
Answer:
A) Fe2O3.XH2O
Question 83.
Which of the following is used as reducing agent in metallurgical process.
A) Coke
B) O2
C) KMnO4
D) None of these
Answer:
A) Coke
Question 84
Which of the following represents calcination ?
im
Answer:
A
Question 85.
Rusting of iron is ……………..
A) reduction process
B) oxidation process
C) chemical decomposition
D) none of these
Answer:
B) oxidation process
Question 86.
A common metal which is highly resistant to corrosion is ……………..
A) Magnesium
B) Aluminium
C) Copper
D) Iron
Answer:
B) Aluminium
Question 87.
Steel is an alloy of ……………..
A) iron and aluminium
B) carbon and manganese
C) zinc and iron
D) iron and carbon
Answer:
D) iron and carbon
![]()
Question 88.
Match the following:
a) Magnesite — i) Ca
b) Horn silver — ii) Mg
c) Lime stone — iii) Fe
d) Haematite — iv) Ag
A) a → i, b → ii, c → iii, d → iv
B) a → iv, b → iii, c → ii, d → i
C) a → ii, b → iv, c → i, d → iii
D) a → iv, b → ii, c → i, d → iii
Answer:
C) a → ii, b → iv, c → i, d → iii
Question 89.
Match the following:
a) Sulphate ore — i) Magnasite
b) Carbonate ore — ii) Epsum salt
c) Oxide ore — iii) Rock salt
d) Chloride ore — iv) Haematite
A) a → i, b → ii, c → iii, d → iv
B) a → iv, b → iii, c → ii, d → i
C) a → iii b → ii, c → i, d—iv
D) a → ii, b → i, c → iv, d → iii
Answer:
D) a → ii, b → i, c → iv, d → iii